INTRODUCTIONMyiasis is the infestation in live vertebrates by Diptera larvae that are fed by live and dead tissues of the host, body liquid substances or the ingested food.
Etymologically, the term myiasis means myie (fly), ase (disease).
It may be divided based on the site of affection in cutaneous, subcutaneous and cavitary (nose, mouth, paranasal sinuses, ocular, vaginal and anal). As to biological characteristics of the fly, it may be mandatory (primary myiasis), in live vertebrates; facultative (secondary myiasis), in necrotic tissues of alive host or in decomposition organic matter; and pseudomyiasis (accidental myiasis), resultant from the ingestion of larvae with food, which go through the intestine without dissolving, leading to possible disorders5.
Nasosinusal myiasis is rare in humans. It is an opportunist disease, caused by non-pathogenic agents that in situations of general organic deficiency or local tissue lesions become harmful4. It is more prevalent in hot and humid climates of tropical countries, where flies are more active and there is higher population density, aggravated by low social-economic conditions and poor hygiene, normally affecting patients who live in rural areas.
Any age range can be affected and it is more common in middle-aged to elderly subjects. Both genera can be equally affected.
The predisposing factors are chronic catarrhal rhinitis, syphilitic rhinitis, Hansen disease, tuberculosis, rhinoscleroma and atrophic or ozenous rhinitis. It is the most frequent factor that reduces the sensitivity of nasal mucosa, expands the nasal cavity, produces fetid odor crusts and thick purulent discharge, factors that attract flies to lay their eggs while the host sleeps. The hatched larvae migrate to the nasal cavities and paranasal sinuses, where they develop by feeding from mucous secretions. They may also intensify secretions by destroying the mucosa membrane that recovers the cavities, cartilages and bones, progressing to invasion of meninges and brain in more advanced stages of the disease, which could result in death.
Signs and symptoms normally observed are epistaxis, nasal obstruction, nasal pruritus, sneezes, purulent rhinorrhea, sensation of foreign body moving in the nose, fetid odor, headache, facial edema and dysphagia. Some patients reported discharge of larvae through the nose and mouth. The examination shows presence of mobile larvae in the nasal cavities and paranasal sinuses, with necrosis of mucosa and osteocartilaginous skeleton.
Review of literature and differential diagnosis
Hungria4 reported that myiasis is a pathology caused by larvae of fly genus Cochilioma macellaria. Treatment consists of removal of larvae with clamp and previous use of calomel or iodoform.
Sharma8, in a 10-year retrospective study, presented 252 cases of nasal myiasis. He reported that in his region, the larvae of the fly responsible for nasal myiasis belonged to the genus Chrysomia bezziana, and most of his patients (97%) had atrophic rhinitis as the most frequent predisposing factor. Treatment conducted with nasal packing soaked in chloroform and terebinthine, followed by manual removal of larvae, was effective. In cases in which patients rejected surgery, there was recurrence of the disease in 81% of the cases.
Agrawal1 reported a case of nasal myiasis caused by Oestrus ovis in a HIV patient, emphasizing that infestation in humans may take place in the urban area and that treatment with chloroform and terebinthine was ineffective.
Quesada7 reported a case of nasal myiasis in the urban population. He reported that human infestation by Oestrus ovis was not part of the normal cycle of the host and it was self-limited, since the larvae did not develop beyond the first stage, resulting in spontaneous expelling with no need for further treatment approaches.
Popov6 reported that sneezes were early and constant symptoms owing to the sensation of the movement of the larvae and that they should always be valued. Chloroform is the most satisfactory treatment because it kills the larvae in 30 seconds, but nasal lavage should be avoided because the water flow may take the eggs into other spaces.
Lucientes3 reported one case in which Oestrus ovis infection affected a eutrophic patient and the larvae developed up to the third larva stage; according to the author, the complaint of foreign body movement in the nasal cavity should always be investigated, especially in tropical countries.
Smith9 reported cases of nasal myiasis in humans caused by larvae genus Cochliomya macellaria, which took three to six days to develop. The author also noticed that infestation was more frequent in summer because of the increased number of flies.
Cabrera2 reported success in the treatment of superficial myiasis with Ivermectine, a synthetic antiparasitic drug, derived from pentacycline lactone, that acted by opening chlorine channels and causing flaccid paralysis by irreversible blockade of synapses between central neurons and motoneurons of invertebrates. This drug has been used since 1987 by WHO to treat parasitosis. According to the author, it is an excellent therapeutic option for the treatment of myiasis.
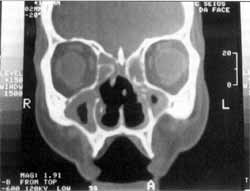
Figure 1. CT scan showing osteocartilaginous destruction of nasal septum, velamentum of paranasal sinuses, no foreign bodies.
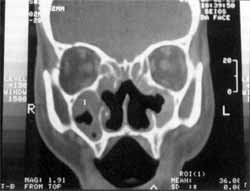
Figure 2. CT scan showing erosion of medial wall of maxillary sinuses, unciform processes and concha bone laminae.
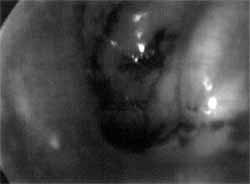
Figure 3. Nasal endoscopy showing larvae of the fly Cochliomyia hominivorax in the left nasal fossa, migrating into the rhinopharynx.
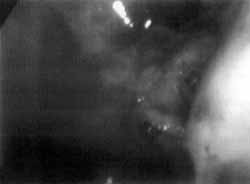
Figure 4. Nasal endoscopy showing larvae of flies infiltrated in the eustachian tube and left nasal fossa.
The present article aimed at presenting a clinical case of a 51-year-old male patient, married, born and living in Caieiras/SP, who lived in a country house in the rural area. For four days he presented epistaxis and sneezes associated with discharge of larvae through the nose and mouth. He reported constant nasal obstruction. He reported a similar episode one year before, when he was submitted to nasal endoscopic surgery to remove the larvae, remaining asymptomatic after that.
At physical examination and nasal endoscopy, we noticed congestion and edema of the nasal mucosa with catarrhal secretion, necrotic material and innumerous larvae discharging from the nose, septum perforation, and ulceration of the lateral walls of the nasal cavity and eustachian tube. Computed tomography without contrast showed velamentum of paranasal sinuses, no foreign bodies, bone failures of nasal septum, medial wall of maxillary sinuses, unciform process and concha bone lamina. Bone thickening of anterior, posterior and lateral walls of maxillary sinuses, suggestive of chronic inflammatory process (Figures 1 and 2).
The patient was submitted to nasal endoscopic microsurgery for removal of larvae from the nasal fossa, maxillary sinuses, eustachian tube, posterior rhinopharyngeal wall and tonsils. The larvae had destroyed the bone structures and were infiltrating the soft parts, impairing their removal (Figures 3 and 4).
After the surgical act, we performed volemic reposition and parenteral antibiotic therapy to control the associated infection. The removed material was placed in chloroform and sent to analysis at Instituto Biológico de São Paulo - Centro de Sanidade Vegetal, which identified 200 larvae of the species Cochliomyia hominivorax, part of the family Calliphoridae, a Diptera order. The myiasis caused by this species is popularly known in Brazil as "bicheira". Larvae are mandatory parasites and develop in healthy non-necrotic tissues. Females are attracted to lay the eggs in live tissues by the smell of blood or morbid tissues. Larvae from this species originate cutaneous or cavitary myiasis.
Seven days after the surgery, the patient returned to the operating room, we performed bilateral nasal packing soaked in calomel for 30 minutes, followed by new nasal endoscopy, which revealed absence of larvae. We then administered 12mg Ivermectine PO, one single dose at early postoperative, and scheduled ambulatory follow-up one week later. This antiparasitic drug induces paralysis of tonic muscle of larvae, resulting in immobilization and death.
Weekly ambulatory follow-up was conducted for 2 months to remove crusts and proceed nasal hygiene and the patient has remained asymptomatic and with no larvae.
DISCUSSIONIn the medical literature, there are few studies and case reports of nasal myiasis.
The species that cause nasal myiasis in humans vary according to geographical region. Authors have listed larvae of the genus Cochliomya macellaria5,9, Chrysomia bezziana8, Oestrus ovisl,7,3 We detected larvae from the genus Cochliomyia hominivorax in the case reported by the present article. Despite the references of nasal myiasis in urban populations1,7,3, the case reported here was of a man who lived in the rural area, had atrophic rhinitis and low social-economic background, similar to the findings by Sharm8.
We disagree with Popov6 when he says that chloroform is the most satisfactory treatment, killing larvae in 30 seconds. In our clinical study, after the removal of the larvae, they were placed in chloroform and remained mobile for 24 hours.
We agree with Sharm8 about the effectiveness of treatment: it should be proceeded with clamp removal of larvae. Similarly to Cabrera2, we do believe that associated use of a synthetic antiparasitic drug is useful, because it enables rupture of the larvae cycle and prevents the hatching of new eggs of remaining larvae.
FINAL COMMENTSNasal myiasis is rare in urban centers and the few cases seen in urban services are of people coming from the rural areas. In our case, the fly larvae were genus Cochliomyia hominivorax, family Calliphoridae.
Treatment is successful after surgical removal of larvae with clamps and oral administration of Ivermectine, used as a boast drug for the larvae that were not identified.
REFERENCES1. AGRAWAL, R. D.; AHLUWALIA, S. S.; BHATIA, B. B. Occurrence of Oestrus ovis larvae in nasal cavity of a woman. J. Indian Med. Assoc., 70 (11): 263, 1978.
2. CABRERA, H.; PIETROPAOLO, N.; ARTO, G. Tratamiento de miasis superficial con Ivermectina. Act. Terap. Dermatol., 21: 370-372, 1998.
3. LUCIENTES, J.; CLAVEL, A. - One case of nasal human myiasis caused by third stage instar larvae of Oestrus ovis. Am. J Top. Med. Hyg., 56 (6): 608-69, 1997.
4. HUNGRIA, H. - Otorrinolaringologia, 8ª edição, Rio de Janeiro, Editora Guanabara Koogan S.A., 2000, 25-28. 5. NEVES, D. E - Parasitologia Humana, 8ª edição, São Paulo, Editora Atheneu, 1991, 401-410.
6. POPOV, C. N. E - Myiasis of the nose. Archives of Otolaryngology, 112-116, 1942.
7. QUESADA, E; NAVARRETE, M. L.; MAESO, J. - Nasal myiasis due to Oestrus ovis larvae. Eur. Arch. Otorhinolaryngol, 247: 131-132, 1990.
8. SHARMA, H.; DAYAL, D.; AGRAWAL, S. E - Nasal myiasis: Review of 10 years experience. The Journal of Laryngology and Otology, 103: 489-491, 1989.
9. SMITH, D. R.; CLEVENGE, R. R. - Nosocomial nasal myiasis. Arch. Pathol. Lab. Med., 110: 439-440, 1986.
* Resident Physician, Núcleo de Otorrinolaringologia de São Paulo.
** Assistant Physician, Núcleo de Otorrinolaringologia de São Paulo.
*** Medical Director, Núcleo de Otorrinolaringologia de São Paulo.
Address correspondence to: Dr. José Antonio Pinto - Alameda dos Nhambiquaras, 159-Indianápolis-04090-010 São Paulo/SP.
Tel/Fax: (55 11) 5573-1970-E-mail: japorl@cepa.com.br
Article submitted on January 30, 2001. Article accepted on March 9, 2001.


