INTRODUCTIONCisplatin is a chemotherapy agent that has been used since the 70's for the treatment of testis and ovarian carcinoma and bone and head and neck tumors. It was created in 1965 by Rosemberg et al.20 who passed an electrical current between two platinum electrodes in a culture of Escherichia coli and observed the inhibition of growth.
Cisplatin renal toxicity is its main toxic effect, which may lead to renal failure. Previous use of hydration and infusion with hypertonic solution of sodium chloride at 3%22, currently used as routine, has contributed to reduce toxicity Myelosuppression is frequently detected, but it may be reversed with interruption of chemotherapy14. Nausea and vomiting are controlled with hydration and the use of anti-emetic drugs. Peripheral neuropathy is also reversible, affecting mainly the limbs and related with accumulated doses.
Ototoxicity is normally irreversible, bilateral, sensorineural and affects mainly the frequencies from 4 to 8kHz; however, with accumulated doses it may progress into speech frequencies3,8,11,17. The incidence of ototoxicity in the literature varied from 12.5%12 to 100%2 in patients submitted to chemotherapy with cisplatin. Age seems to be an aggravating factor of ototoxicity: it is more common in the elderly5,8 and in children4,19,21. Accumulated dose4,8,14 and bolus administration19,23 are factors that aggravate ototoxicity.
Histologic findings in humans showed destruction of three rows of outer hair cells, specially on cochlear spiral basal, with slight interference on inner hair cells6,7.
The present study aimed at assessing hearing of children who used cisplatin as chemotherapy agent.
MATERIAL AND METHODWe evaluated eight children who used cisplatin, mean age of 10.2 years, from August 1996 to March 1999. All patients were treated at Hospital Sarina Rolim Caracante, in the city of Sorocaba/SE. There were four male and four female subjects. The chemotherapy protocol used was similar to the one used at the Unity of Oncologic Hematology at Instituto da Criança, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. The initial individual cisplatin dose was 90 mg/m² and the total dose varied from 180 to 630 mg/m². As to tumor history, there were two patients with osteosarcoma, two cases of rhabdomyosarcoma, two cases of Hodgkin lymphoma, one case of medulloblastoma and one case of mesenchymal chondrosarcoma.
The ENT exam was conducted by the same examiner and the patients were evaluated on auditory history before chemotherapy and previous history of ENT infections. Audiological assessments were conducted by the same examiner, in a sound-proof booth, using audiometer MA-41 by Maico and middle ear analyzer MA630 by the same manufacturer. All patients underwent pure tone audiometry in frequencies 250Hz to 8kHz, speech recognition index (SRI), immitanciometry and acoustic reflex. Thresholds equal or below 20dbHL in the tested frequencies were considered within the normal range.
RESULTSWe conducted 28 audiological assessments overall, but in two patients only one assessment was performed after the end of chemotherapy. In Table 1 we can see tumor histology and pathology, dose of cisplatin and audiometric results of each patient. Tympanometry was normal in all cases. Acoustic reflexes were higher in the affected frequencies. Speech recognition index was compatible with the results from pure tone audiometry.
Owing to difficulties in speech discrimination and school problems, one patient was referred to hearing aid fitting.
DISCUSSIONOtotoxicity of cisplatin was initially reported by Rossof et al., in 1972, and it has been studied throughout the ears. The incidence of ototoxicity is widely variable in the literature, affecting from 12.5%12 to 100%2, 9, and it is directly related to dose and administration route of cisplatin. In our study, only one of the eight patients did not have hearing loss after the use of cisplatin. The hearing loss is initially located between 6 and 8 kHz, and as a result of accumulated doses, it may affect frequencies below 4kHz10,19. Symmetry of hearing loss is a common finding, according to the literature, and in our study we found asymmetry in one patient. This finding may be related to local radiotherapy, applied on the cranial region of the patient. In our study, frequencies of 6 and 8kHz were the most affected ones and, as a result of accumulation doses, there was the affection of other frequencies. Irreversibility is a common finding; we noticed in our study that the patients who underwent hearing assessment few days after chemotherapy showed improvement of hearing in subsequent assessments. We believe that this finding may be a result of the poor clinical conditions of the patients during the assessments.
Age is an aggravating factor of ototoxicity of cisplatin, and therefore, children4,19,21 and the elderly 5, 8 are more predispose to it. The use of previous or concomitant radiotherapy with cisplatin is also a factor that worsens the hearing status12,21. In our study, only one patient had radiotherapy on the cranial region - he experienced worsening of hearing on the side close to the irradiated area.
TABLE 1 -Histopathology, dose of cisplatin and audiometric results.
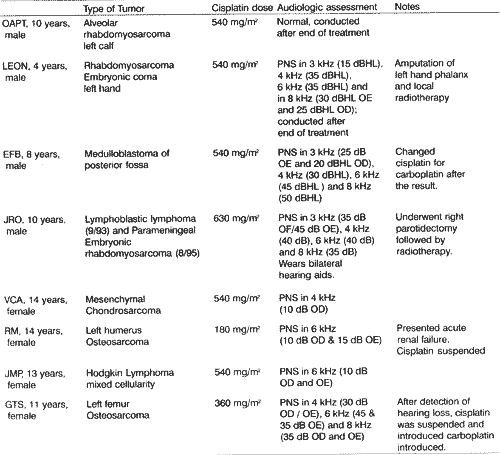
Key: PNS: sensorineural loss; OE: left ear; OD: right ear.
Pure tone audiometry is the most common method used to diagnose ototoxicity of cisplatin. In children, visual reinforcement audiometry4,15, brainstem evoked audiometry2,4,13, or otoacoustic emissions13,18, la may be used to detect ototoxicity. High frequency audiometry is a very effective method to detect early cisplatin-induced ototoxicity5,13,24, because early losses have been observed in frequencies between 12 and 20kHz. ASHA (American Speech-Language-Hearing Association)1 advocates assessment of patients up to 24 hours before the use of cisplatin and 24 hours within the doses, but frequency of assessment may vary denendina on the alterations found.
CONCLUSIONCisplatin is an ototoxic chemotherapy agent that produces symmetrical, irreversible high frequency loss. Pretreatment assessment and assessments before each dose are essential to guarantee early diagnosis of hearing loss. The possibility of changing the drug when there is significant hearing loss and impairment of speech indexes should be discussed with the otorhinolaryngologist. The use of hearing aids and speech and hearing follow-up should take place as early as possible, especially in children at language acquisition and school age.
REFERENCES1. AMERICAN SPEECH-LANGUAGE-HEARING ASSOCIATION - Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. Asha, 36 11-19, 1994.
2. BARB HAMITON, R. M.; MATHELSON, L. M.; KEAY, D. G. - Ototoxicity of cisplatinum and its relationship to eye color. J Laryngol Otol., 105: 7-11, 1991.
3. BLAKLEY, B. W; GUPTA, A. K.; MYRES, S. E ; SCHWAN, S. - Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg., 120: 541-6, 1994.
4. BROCK, P R.; BELLMAN, S. C.; YELMANS, E. C.; PINKERTON, C. R.; PRICHARD, J. - Cisplatin ototoxicity in children: a practical grading system. Med Pediatric Oncol.; 19: 292-300, 1991.
5. DRESCHLER, W A.; HULST, R. J.; TANGE, R. A.; URBANUS, N. A. - The role of high-frequency audiometry in early detection of ototocity. Audiology, 24: 387-95, 1985.
6. FAUSTI, S. A.; HENRY, J. A.; SCHAFFER, H. L; OSLON, D. J.; FREY, R. H.; BAGBY, G. C. JR. - High frequency monitoring for early detection of cisplatin ototoxicity Arch Otolaryngol Head Neck, 119: 661-6, 1993.
7. FAUSTI, S. A.; SCHECHTER, M. A.; RAPPAPORT, B. Z.; FREY, R. H.; MASS, R. E. - Early detection of cisplatin ototoxicity Cancer, 53: 224-31, 1984.
8. HELSON, L.; OKONKWO, E.; ANTON, L.; CVITKOVIC, E. - Cisplatinum ototoxicity Clinical Toxicology; 13: 469-478, 1978.
9. HINOJOSA, R.; RIGGS, L. C.; STRAUSS, M.; MATZ, G. J. - Temporal bone histopathology of cisplatin ototoxicity. Am J. Otol., 16 731-40, 1995.
10. KALAMAR, E; FREEMAN, A. L; HIGBY, D. J.; WALLACE, H. J. JR.; SINKS, L. F. - Clinical response and toxicity with cis-dichlorodiammineplatinum (II) in children. Cancer Treat Rep., 61: 835-9, 1997.
11. KOMUNE, S.; ASAKUMA, S.; SNOW, J. B. JR. Pathophysiology of the ototoxicity of cis-diamminedichloroplatinun. Otolaryngol Head Neck Surg., 89: 275-82, 1981.
12. KOPELMAM, J.; BUDNICK, A. S.; SESSIONS, R. B.; KRAMER, M. B.; WONG, G. Y - Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope, 98: 858-64, 1988.
13. KRETSCHMAR, C. S.; WARREN, M. E; LAVALLY, B. L.; TARDELL, N. J. - Ototoxicity of pre-radiation cisplatin for children with central nervous system tumors J Clin Oncol, 8: 1191-8, 1990.
14. LIPPMAN, A. J.; HELSON, C.; HELSON, L. - Clinical trials of Cis-diamminedichloroplatinun (NSH-119875). Cancer chemother Rep., 57: 191-200, 1974.
15. MIETTINEN, S.; LAURIKAINEN, E.; JOHANSSON, R.; MINN, H.; LAURELL, G.; SALMI, T. T. - Radiotherapy enhanced ototoxicity of cisplatin in children. Acta Otolaryngol (Stock), supl 529: 90-4, 1997.
16. PASIC, T R. & DOBIE, R. A. - Cisplatinun ototoxicity in children. Laryngoscope, 101: 985-91, 1991.
17. PIEL, I. J.; MEYER, D.; PERIIA, C. E; WOLFE, V I. Effects of cis-diamminedichloroplatinun on hearing function in man. Cancer chemother Rep., 58, 871-5, 1974.
18. PLINKERT, E K. & KRÖBER, S. - Früherkennung einer cisplatin ototoxizität durch evozierte otoakustishe emissionen. Laryngorhinootologie, 70: 457-62, 1991.
19. REDDEL, R. R.; KEFFORD, R. F.; GRANT, J. M.; COATES, A. S.; FOX, R.M.; TATTERSALL, M.H. - Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep., 66: 1923, 1982.
20. ROSENBERG, B.; VANCAMP, L.; KRIGAS, T. - Inhibition of cell division in Escherichia Coli by electrolysis products from a platinum electrode. Nature, 205: 689-9, 1965.
21. SCHELL, M. J.; MCHANEY, V A.; GREEN, A. A.; KUN, L. E.; HAYES, F. A.; HOROWITS, M.; MEYER, W H. - Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol., 7: 75460, 1989.
22. SCHWEITER, V G. - Ototoxicity of chemotherapeutic agents. Otolaryngol Clin North Am., 26: 759-89, 1993.
23. STRAUSS, M.; TOWFIGHI, J.; LIPTON, A.; BROWN, B.; LORD, S.; HARVEY, H. A. - Cis-platinum ototoxicity: clinical experience and temporal bone histopathology. Laryngoscope; 93: 1554-9, 1983.
24. ZOROWBA, E G.; SCHIMITT, H. J.; GUTJAHR, E Evoked otoacoustic emissions and pure tone threshold audiometry in patients receiving cisplatinun therapy. Int. J Pediatr Otorhinolaryngol.; 25: 73-80. 1993.
* Master in Otorhinolaryngology; Doctorate studies under course, Faculdade de Ciências Médicas da Santa Casa de São Paulo.
** Master in Speech and Hearing Pathology, Pontifícia Universicade Católica de São Paulo.
*** Assistant Instructor at Faculdade de Ciências Médicas a Biológicas, Pontifícia Universidade Católica de São Paulo.
**** Faculty Professor of the Discipline of Otorhinolaryngology, Faculdade de Ciências Médicas da Santa Casa de São Paulo.
Address correspondence to: Godofredo Campos Borges - Rua Francisco Ferreira Leão, 183 - 18040-330 Sorocaba /SP - Tel: (55 15) 722-2657 - Fax: 222-3899.
E-mail: gcborges@uol.com.br
Article submitted on September 14, 2000. Article accepted on February 9, 2001.


