INTRODUCTIONNecrotizing external otitis (NEO) known in the past as malignant external otitis, is a potentially fatal infection that normally starts at the external acoustic canal and extends to the skull base, occurring especially in diabetic elderly patients and despite prolonged antibiotic therapy it is associated with high morbidity and significant mortality 1. We present the case of an adult diabetic patient with NEO and bilateral peripheral facial palsy that progressed to cure of the infection, but without improvement of facial palsy. The first report of temporal bone osteomyelitis was described in Paris by Telmouche in 1838.2 Later, in 1959, Meltzer and Keleman described progressive osteomyelitis of the temporal bone, zygoma, mandible and skull base caused by Pseudomonas aeruginosa3, but it was Chandler in 1968 that described this clinical entity in its original series of 13 patients, most of them diabetic subjects 4.
REVIEW OF THE LITERATUREAs previously referred, initial terminology of the disease was external malignant otitis, a term initially used by Chandler, being that the term necrotizing was introduced in 1973 by Evans and Richards, providing more precise terminology to the disease. Cohn added the term progressive and Doroghazi replaced "necrotizing" for "invasive". It would be better if only one single term would be consistently maintained in the literature. However, objections to the term malignant are acceptable since it is not neoplasm; even so, the most commonly used term is still malignant external disease. The use of the terms otitis and external is not precise since the infection extends to the deeper plans of the temporal bone, and therefore, it is not only otitis and it is not restricted to the external auditory canal 1, 5, 6.
The causing agent of NEO is most of the times Pseudomonas aeruginosa, which is a mandatory non-sporogenic gram-negative aerobe with polar flagella 7. The most virulent strains produce a mucoid membrane that acts as a protection against phagocytes and antibiotics. This layer is also rich in lithic enzymes responsible for typical necrotizing vasculitis in infections by Pseudomonas aeruginosa. Some strains also produce neurotoxins that can probably have a role in peripheral cranial neuropathy 1, 7. Pseudomonas aeruginosa can be found in about 96 to 98% of the cases of NEO 8. Other pathogens can be eventually identified, such as Aspergillum fumigatus, Proteus mirabilis, Staphylococcus aureus, Staphylococcus epidermidis, Candida albicans, Klebisiella sp.1,6,7,9 . Since Pseudomonas aeruginosa rarely causes infections in healthy tissues, but normally progresses through small lesions, it has been recently detected that in more than 50% of the cases of NEO there are previous maneuvers (trauma and/or iatrogenia) of the external acoustic canal (EAC) that causes the referred lesions 6. Initially, the process is limited to the EAC and then it extends to the osteo-cartilaginous junction towards the temporal bone. The infection passes through the Santorini fissures in the cartilaginous external canal to the mastoid. The facial nerve (7th cranial nerve) normally is reached close to the emergence of the stylomastoid foramen. The infection can progress towards the skull base and affect the glossopharyngeal (9th nerve), vagus (10th nerve) and accessory (11th nerve) nerves causing the jugular foramen syndrome and eventually affecting the hypoglossus nerve (12th nerve). Less frequently, the abducens (6th nerve) and trigeminal (5th nerve) nerves can be affected at the petrous apex. There is a case report in which the optical nerve was affected in a case of NEO 1, 6, 7, 10.
The precise pathogenesis of NEO is unknown. Probably it depends on the combination of two factors such as underlying alterations of the immune system and peculiar characteristics of the bacteria that can be found in this environment of conditions prone to pathogenic activity. Some few cases of NEO are related with other immunosuppression status such as hematological diseases (leukemia and severe anemia), during the treatment with cytotoxic or immunosuppressive drugs, AIDS or positive HIV patients 11-13. There were no cases of diabetic ears that were more colonized than non-diabetic ears with P. aeruginosa. The initial clinical picture is not characteristic and normally starts similarly to acute diffuse external otitis, which tends to delay the correct diagnosis and the beginning of the appropriate therapy. The most frequent symptom (90% of the cases) is otalgia, normally severe and resistant to analgesics, irradiating to the frontal-temporal and parietal regions and worsening at night. In 45% to 100% of the cases, it is followed by fetid and purulent otorrhea. There is mild conductive hearing loss resulting from the obstruction of the external acoustic canal and middle ear secretion. Normally, there are no systemic signals such as fever or tachycardia. Sensitivity and peri-auricular edema are common findings. Another frequent finding is granulation tissue that is located in the external auditory canal, which reveals chronic non-specific inflammatory process. The tissue should be submitted to routine biopsy because even though it is rare it can be diagnosed as squamous cell carcinoma of the external ear in some patients and clinically it is undistinguishable from NEO 14, 15.
There is edema and hyperemia of the EAC, but with intact tympanic membrane 1, 5-7. The associated neurological symptom more frequently seen is 8th nerve paralysis and, as the disease advances, there is skull base osteomyelitis and other nerves are affected, normally in 24 to 43% of the affected patients. There are many complications that can manifest, such as cerebral abscess, sphenoid sinusitis, mycotic aneurysm, paratiditis, lateral and sigmoid sinus thrombosis, followed by high mortality. Normally, the pneumatized areas of the temporal bone, the otic capsule and the membranous labyrinth are spared. The most constant and significant laboratory finding is erythrocyte sedimentation rate (ESR), in addition to hyperglucose level and reduced tolerance to glucose. There may also be moderate leukocytosis 6, 7.
Radiological findings comprise simple x-rays of the mastoid that can show pneumatization hypotransparence or bone destruction, but it is not useful when compared to computed tomography scan (CT). Temporal bone CT with contrast shows thickness of soft tissues and opacification of the mastoid, in addition to subtle changes such as EAC wall erosion before the involvement of temporomandibular joint (TMJ), sequestration of EAC and skull base erosion 5, 7. Magnetic resonance imaging (MRI) can be advantageous in defining the medial extension of the infection towards the skull base, but it does not provide information about the bones. Owing to the persistence of images in CT and MRI even months after clinical improvement, it is necessary to find other ways to assess the cure criteria; authors used scintigraphy with technetium and gallium 7. Scintigraphy with technetium- 99 is useful in the diagnosis of osteitis. The exam is positive in acute and chronic osteomyelitis, as well as in areas of trauma, which reveals its low specificity. Gallium-67 incorporates to proteins and polymorphonuclear bodies in infection sites forming complexes with lactoferrin. It shows the focus of acute infection, but not the whole extension of the osteomyelitis process. As time goes by, scintigraphy with gallium-67 can restore normal values, whereas scintigraphy with technetium -99 can take longer to restore normal values. Therefore, it is recommended initial investigation with technetium and gallium and, afterwards, sequential studies with gallium to monitor response to therapy 7.
There are no universally accepted criteria to classify NEO, but there are three classifications proposed in the literature by Corey et al., Benecke and Levenson et al.. They basically take into account the different grades of disease extension and scintigraphy findings (Figure 1) 7. The diagnosis is based on clinical history, physical examination and laboratory tests. There are four cardinal points in the diagnosis and clinicians should always be highly suspicious so that patients can still receive the appropriate aggressive treatment for the case. The points are: 1. persistent otalgia for over one month; 2. Purulent persistent otorrhea associated with granulation tissue for many weeks; 3. Diabetes mellitus, advanced age or other immunosuppressant state; and 4. involvement of the cranial nerves. We should also conduct culture of the external auditory canal and it frequently has positive result for Pseudomonas aeruginosa.
Antibiotic therapy should be preferably discussed with an infectologist for final definition of the drug. In the literature, we found many possibilities such as azlocilin and mezlocillin, ceftazidime, imipenem, aztreonam, amicacin, norfloxacin and ciprofloxacin. Normally, two antibiotics are associated, one aminoglycoside such as gentamicin or tobramicin (reserving amicacin for resistant strains of Pseudomonas) associated with an anti-pseudomonas antibiotic, which is an old combination, but one that has good results. Initial therapy should be changed depending on the result of the culture. We can use ticarcillin associated with clavulanic acid that combines its anti-pseudomonas effect with the anti-beta-lactamase producer activity and one aminoglycoside. The disadvantages are the nephrotoxic and ototoxic effects of aminoglycosides when used during a very long period of time. Anti-pseudomonas antibiotics have the possibility of causing sodium retention, potassium depletion, hypertransaminasemia, spontaneous bleeding, hemolytic anemia and seizures, requiring mandatory heart monitoring and restricted intake of sodium 5, 6, 7. Ceftazidime is a third-generation bactericidal beta-lactamase resistant cephalosporin that is very active against Pseudomonas and other gram-negative bacteria, better than anti-pseudomonas penicillin, which can be as effective as the association of penicillin and aminoglycoside. It can be used isolated or associated with tobramicin and serves when there is a gap in the use of the anti-pseudomonas penicillin and aminoglycoside combination. The advocated dose is 2g TID. It penetrates the central nervous system when there is inflammation. Cefoperazone is also a third-generation cephalosporin with anti-pseudomonas activity that is a valid option of treatment. We can also make use of beta-lactam antibiotics such as imipenem and aztreonam that are more powerful than anti-pseudomonas penicillin and equivalent to third generation cephalosporin. Resistance can be developed during treatment with Pseudomonas if it is used in monotherapy, which does not happen with aztreonam. It has an additional advantage because it is very safe in patients allergic to penicillin. In addition, they are not ototoxic 7. Recently, some patients had good results with oral use of ciprofloxacin, a fluoroquinilone that acts against Pseudomonas aeruginosa and whose action mechanism is inhibition of the subunit A of bacterial DNA-girase (an enzyme involved in the duplication of bacterial DNA). The dose is 750mg BID and it can be used isolated or in combination with ceftazidime or rifampicin. It has been frequently used as primary home therapy or following the intravenous antibiotic therapy regimen. The side effects are nausea and vomiting, headache and loss of balance, in addition to pruritus, rash and skin eruptions. Ciprofloxacin can be used for a long period of time of up to six months after the end of intravenous therapy. There has about 20% resistance according to reports in patients that used it for long periods of time (six weeks or more) in management of osteomyelitis. The drug should not be used in patients younger than 18 years, since there is evidence of arthropathy of joints in immature animals in the growing period. The patients should be treated with antibiotics for a long period of 4 to 8 weeks, average of six weeks. The treatment duration, however, is empirical and to present there is no consensual period in the literature.
Some of the proposed criteria of cure were skin healing of EAC and negative culture. More recently, normal values of erythrocyte sedimentation rate were suggested as an acceptable and safe criterion. Others consider that only the restoration to normal levels presented in the scintigraphy with Gallium -67 should be used to discontinue antibiotics. An important signal that treatment is effective is reduction or interruption of ear pain, which sometimes leads the patient to considering treatment discontinuation. There is no indication to the use of otological drops of gentamicin or ciprofloxacin in the EAC as in diffuse external otitis, since it is the inappropriate route to administer antibiotics to an infection that is deep into the ear, and it hinders the collection for cultures 1, 5-7.
Most of the patients can be treated clinically and the role of surgery is still controversial. Surgical debridment of devitalized tissues and the bone affected by osteomyelitis is made only in patients in which there is no response to conventional clinical treatment. There are signals suggestive of higher likelihood of surgical treatment, such as progressive pain that does not respond to medication, development of cranial neuropathy and persistence of granulation in the EAC. There are reports of surgical procedures in which local extensive excision of the affected cartilages and devitalized tissues is made, as well as the extensive resection of the bone to have access to the primary focus and promote the appropriate drainage 1. Mortality continues to be significant, especially in the group with marked immunosuppression. Meyerhoff et al. referred that mortality before the introduction of carbenicillin and gentamicin was 37% and that after it, it dropped to 23%. The true mortality is even higher since many cases are not even reported 5.
Recurrence is a well-known aspect of the disease, present in 9 to 27% of the patients and manifested initially by insidious otalgia followed by headache and TMJ pain. Surprisingly, otorrhea can be minimal or not show up at all, reflecting the predominance of skull base osteomyelitis as causing the clinical syndrome. The first objective signal is new increase of erythrocyte sedimentation rate.
Diagnosis and treatment of patients with NEO are still a challenge to Otorhinolaryngologists. Probably the main advance in all those years has been the identification of NEO as a different clinical entity and better understanding of its pathophysiology. If there are high level of clinical suspicion, early diagnosis, appropriate treatment and collaboration of the patient, mortality tends to drop.
CASE REPORTJES, 70 years old Caucasian male, married, resident in Recife/PE, with clinical history of type II diabetes mellitus, came to the Ambulatory of Otorhinolaryngology, UFPE, in December 2000, presenting otorrhea and bilateral peripheral facial palsy. He reported history of a fall of his own height about four months before that progressed to otorrhea and facial palsy, initially on the left and then, after one month, the symptoms started on the right as well. The physical examination showed fetid secretion with areas of granulation tissue on both external auditory canals, and bilateral facial palsy grade five (House Brackmann classification) (Figure 2). He had temporal bone CT scan conducted two months before that evidenced presence of hypodense material comprising the mastoid antrum and tympanic cavities bilaterally, with small osteolitic lesions involving the tip of the mastoid on the left. The patient was hospitalized and the admission tests showed fast glucose 436 mg/dl (normal: 70 - 110 mg/dl), BUN 24 mg/dl (normal: 8-20 mg/dl), creatinine 1.2 mg/dl (normal: 0.6 - 1.2 mg/dl), Na 133 meq/l (normal 135 - 145 meq/l), K 3.7 meq/l (normal: 3.5 - 5.0 meq/l), complete blood count with leukocytosis of 18,300/mm3 (normal 5000 - 10000/mm3) without deviation to the left. We suspected of NEO and started treatment with Ceftazidime, one intravenous gram BID and Gentamicin, 80 mg IV TID, correction of glucose levels with insulin and oral hypoglucose drugs and follow up with the endocrinology area. On the 6th hospitalization day, he presented discharge through the external auditory canals and leukocyte count of 14,600/mm3 without deviation to the left. The antibiotic regimen was maintained for two weeks, but the patient developed acute renal failure and the nephrology team suggested change of the antibiotic regimen owing to the possibility of having this manifestations as secondary to aminoglycoside use or a possible infectious cause. The antibiotic regimen was replaced for ciprofloxacin (400 mg IV BID) which was used for two more weeks, completed by 8 weeks of treatment with the same drug PO (500mg BID). During hospitalization, we conducted motor physical therapy for facial palsy. Renal failure was clinically compensated. Pure tone audiometry showed right ear (RE) mixed profound hearing loss and on the left (LE) mild conductive hearing loss. The patient progressed with improvement of the clinical condition and absence of otorrhea and granulation in the EAC with bilateral intact tympanic membrane of normal aspect. We conducted scintigraphy with gallium after two months from the beginning of treatment, suggesting an infectious process on the RE (Figure 3 and 4). The control temporal bone CT scan after two months did not show signals of active disease.
CLOSING REMARKSThe authors described an atypical presentation of NEO, affecting both temporal bones and bilateral peripheral facial palsy. Differential diagnosis with neurological diseases (peripheral motor neuron syndrome), systemic disease and manifestation secondary to trauma was made, but they were all ruled out owing to the clinical findings, complementary tests and progression of the pathology, concluding that facial palsy was owed to the temporal bone infectious process. Ideally, we should conduct Tc99m and gallium 67 scintigraphy, as well as erythrocyte sedimentation rate at the beginning of diagnosis and management. However, since the procedures were not readily available, they were not performed. The clinical treatment proposed was the use of anti-pseudomonas cephalosporin (initially ceftazidime associated with gentamicin, and later ciprofloxacin) for a period of 8 weeks, being that the latter was used as continuous oral treatment. There was no need for surgical debridment. Glucose levels were only appropriately controlled once the infectious process improved. We cannot confirm for sure that the scintigraphy with gallium 67 conducted two months after the end of treatment really translated the worsening of the infectious process, since it would be essential to compare it to baseline tests. Erythrocyte sedimentation rate in the same period was 8mm/h (normal: male <10mm/h). The results of ESR and absence of signals of osteitis in the temporal bone CT scan allowed us to conclude that clinical cure was achieved since the patient has been free from the disease for one year now.
Figure 1
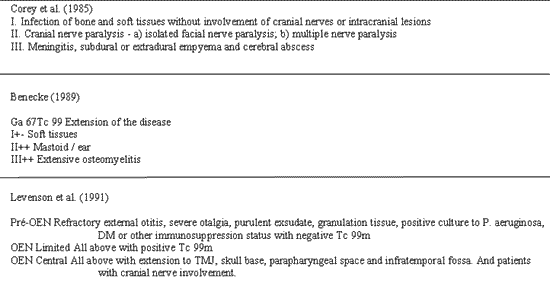

Figure 1. Classifications proposed for necrotizing external otitis.
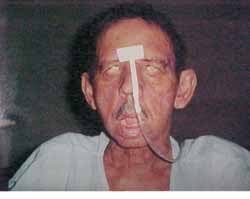
Figure 2. Patient with bilateral peripheral facial paralysis grade V (House-Brackmann).
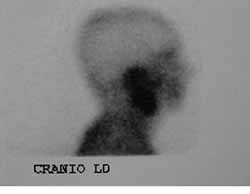
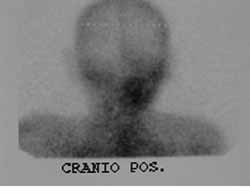
Figures 3 and 4. Scintigraphy with Galium-67 demonstrating hypercaption of contracts at the level of the right ear.
1. Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations and therapy. The American Journal of Medicine 1998; 85:391-8.
2. Meltzer PE, Kellemen G. Pyoceaneous osteomyelitis of the temporal bone, mandible, and zygoma. Laryngoscope 1959; 169:1300-16.
3. Strauss M. Current therapy of malignant external otitis. Arc Otolaryngol Head Neck Surg 1990; 102 (2): 174-6.
4. Chandler JR. Malignant external otitis, Laryngoscope 1968; 78:1257-94.
5. Matucci KF, Setzen M, Galantich P. Necrotizing otitis externa occurring concurrently with epidermoid carcinoma. Laryngoscope 1986; 96:264-7.
6. Grandis JR, Curtin HD, Yu VL. Necrotizing (malignant) external otitis: prospective comparison of CT and MR imaging in diagnosis and follow up. Radiology 1995; 196: 499-504.
7. Amorosa L, Modugno GC, Pirodda. Malignant external otitis: review and personal experience. Acta Otolaryngol (Stockh) 1996; 521:3-16.
8. Ennouri A et al. Les otites externes malignes du duabetique (Malignant external otitis in diabetic patients). Revue de Laryngologie 1989; 110:9-12.
9. Barrow HN, Levenson MJ. Necrotizing "malignant" external otitis caused by Staphylococus epidermidis. Arc. Otolaryngol Head Neck Surg 1992; 118:94-6.
10. Bath AP, Rowe JR, Innes AJ. Clinical records: malignant otitis externa with optic neuritis. The journal of laryngology and otology 1998; 112:274-7.
11. Weinroth SE et al. Malignant otitis externa in AIDS patients: Case report and review of the literature. ENT Journal 1994: 772-8.
12. Al-Shihabi BA. Carcinoma of temporal bone presenting as malignant otitis externa. The Journal of Laryngology and Otology 1992; 106:908-10.
13. Mello LRP et al. Otite externa necrotizante em paciente pediátrico imunodeficiente por desnutrição. Revista Brasileira de ORL 1997; 63 (5): 500-4.
14. Nir D et al. Clinical records malignant external otitis in an infant. The Journal of Laryngology and Otology 1990; 104:488-90.
15. Rubin J et al. Malignant external otitis in children. The journal of Pediatrics 1998; 113(6): 965-9.
1 Substitute Professor (Master in Surgery) and Preceptor, Department of Otorhinolaryngology, HC-UFPE.
2 Substitute Professor, Department of Otorhinolaryngology, HC-UFPE.
3 Resident Physician.
4 Faculty Professor, Department of Otorhinolaryngology, HC-UFPE.
Address correspondence to: Dr. Antonio Antunes - Rua Paissandú 381/1001 Boa Vista Recife PE 50070-200
Tel (55 81)3432-1270 - Fax (55 81)343-13667 - E-mail: aantunes@truenet.com.br
Study conducted at the Discipline of Otorhinolaryngology, Federal University of Pernambuco, presented at 36º Congresso Brasileiro de Otorrinolaringologia, Florianópolis-SC, held on November 19-23, 2002.


