INTRODUCTIONOtoacoustic emissions are the responses that the cochlea produces in the form of acoustic energy. The recognition that the cochlea did not only receive sounds but also produced acoustic energy was one of the main factors to support the theories of cochlear function.
In 1948, Gold16 proposed the existence of a positive mechanical system of feedback located inside the cochlea, what increased the movement of the basilar membrane, consisting on a process of mechanical-electrical transduction coupled to a process of electrical-mechanical transduction. He previewed in that time the presence of otoacoustic emissions.
Kemp20, in 1978, was the first researcher to confirm the existence of energy that was really produced by the cochlea and that it could be registered as acoustic vibrations of the external ear canal, using the appropriate methods and equipment. They are associated with nonlinear processes present in the normal cochlea, which increase sensitivity and selectivity of frequency of the inner ear. The origin is considered to be associated with mechanical processes of outer hair cells, mediated by the action of efferent auditory nervous pathways.
Since its discovery, innumerous researchers have investigated this phenomenon as a possible base for clinical testing of cochlear function.
Regular clinical application of otoacoustic emissions was designed for assessment of cochlear function, as a method to track hearing loss in newborns, monitor auditory function and investigate cochlear function of subjects exposed to intense noise and in people who made use of ototoxic medication23. In our community, various authors have studied the presence of spontaneous and evoked otoacoustic emissions especially in high-risk newborns15, 24. Many studies have been conducted in patients who have tinnitus but no definite conclusions have been made yet.
Normal auditory function depends on active and passive cochlear mechanisms.
An active process takes place inside the cochlea, as a response to sound stimulation. In this process, there is amplification of sound, and it seems that outer hair cells have a significant participation in this phase. Motility of these cells leads to increase and modulation of the dislocating wave existent in the basilar membrane when stimulated by an acoustic tone.
When the cochlea is stimulated by a sound, as a result of the movement of stapes, a wave propagates through the labyrinthic fluids, causing movement of basilar membrane and consequent dislocation of outer hair cells - initially towards tectorial membrane. Due to the difference in phases between the movements of these two membranes, cilia are flexed, opening ionic channels - potassium channels, in the I stereocilia, leading to depolarization and creating a change in polarity of membranes of outer hair cells. It leads to shortening and elongation of these cells, and in some way, this movement is regressively coupled to the movement of the basilar membrane, which may increase it. Components of this movement, induced by the alteration in length of the outer hair cells, could be somewhat involved in the formation of otoacoustic emissions6, 7, 8.
Differently from what happens with inner hair cells, outer cells have proteins, such as actin, myosin, fibrin and tropomyosin, which are part of muscular contraction13.
It is normally accepted that the movement of outer hair cells is a "cochlear amplifier" and that it controls sensitivity of cochlea10.
Anatomical and physiological evidence supports the interdependent function of both ears coordinated through efferent neural pathways, which connect one side of the hearing system to the other, through medial and lateral components of olivocochlear system. They may be distinguished; either by their projections or the location of their respective neuronal bodies, on the medial and lateral regions of superior olivary complex14, 28, 29.
Medial olivocochlear bundle consists of about 80% of crossed nervous fibers and 20% of ipsilateral fibers, and it projects its endings predominantly towards the contralateral cochlea. They end below the outer hair cells18. This bundle has a population of fibers that is relatively thick and myelinized, and has synapses with these cells. These neurons are located on the medial, ventral or lateral areas of the periolivary zones and they are related with the projection field of the contralateral ventral cochlear nucleus29.
The olivocochlear bundle leaves the brainstem as a component of the lower division of vestibular nerve 29. In the region of sacular ganglion, the fibers leave behind the sacular nerve and form the Oort vestibular-cochlear anastomosis, heading towards the apical region, inside the spiral intraganglionar bundle5, passing radially spirally through the bone spiral lamina. After entering Corti's organ, medial efferent fibers reach the outer hair cells, together with the superior radial fibers of the tunnel (Figure 1)30.
It is observed that the medial bundle has synapses with outer hair cells. Thanks to their activity, both contralateral and ipsilateral sounds affects the activity of these cells, by releasing chemical transmitters, such as acetylcholine13. Therefore, efferent fibers play an important role in the adjustment of the active mechanism that involves outer hair cells4, 21.
The most known efferent effect is the reduction of action potential of auditory nerve. This potential is produced by synchronous discharges of radial myelinized efferent auditory fibers, which innervate inner hair cells, but with no contribution from the spiral non-myelinized afferent auditory fibers that innervate outer hair cells. Therefore, we may consider that the medial efferent system that has synapses with these cells acts over the discharges of afferent auditory fibers that innervate inner hair cells. An explanation for this phenomenon, observed in guinea pigs, may be that the medial efferent system has an effect of depression over the movement of the basilar membrane as a response to sound11, 17.
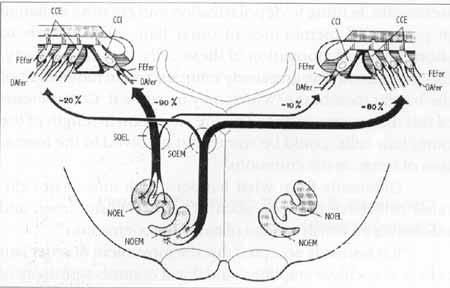
Figure 1. Adapted from Warr et al. 3°, 1986 - Efferent olivocochlear system.
NOEM: Medial efferent olivocochlear nucleus; NOEL: Lateral efferent olivocochlear nucleus; SOEM: Medial efferent olivocochlear system; SOEL: Lateral efferent olivocochlear system; Dafer: afferent dendrites; Fefer: efferent fibers; CCI: inner hair cells; CCE: outer hair cells.
In addition to the inhibitory effect on the action potential of the auditory nerve, the efferent system may also act on cochlear potentials. It causes the increase in amplitude of cochlear microphony26. Cochlear microphony is produced by a movement of sound frequency that changes the resistance of stereocilia of outer hair cells, leading to the production of an electrical current flow through these cells and the cochlea10. Efferent stimulation, by means of increase in basolateral conductance of these cells. and hyperpolarlzation, increases this current flow, thus, increasing cochlear microphony.
In human beings, this hyperpolarization has never been measured; however, in many hair cells of non-mammalians, efferent stimulation increases conductance and hyperpolarizes outer hair cells13.
Many studies have shown the suppression of otoacoustic emissions and inhibition of electrical activity of auditory nerve in animals27 by stimulating electrically the nervous fibers or the nuclei of the efferent nervous system - the same is observed in human beings when suppression of transient and spontaneous emissions takes place as a result of contralateral acoustic stimulation1, 9.
The study of otoacoustic emissions may be performed characterize if the compromise is sensorial or neural, analyzing each side at a time9.
The record of otoacoustic emissions is useful not only the analysis of the integrity of each ear. It may also be used evaluate the interaction between them by studying suppression of emissions, presenting extra stimuli to the same ear, t other ear or to both simultaneously2, 23.
In normal subjects, contralateral stimulation, by means efferent auditory nervous pathways and their action, cold cause an inhibition of cochlear responses, leading to reduction of amplitude of register of otoacoustic emissions19. However; subjects with retrocochlear pathologies there should not be t reduction in record of emissions3.
Based on these data, we defined the objectives of this study as follows:
1) Compare transient and distortion product EOA obtained 1 normal subjects, with and without contralateral stimulation.
2) Compare EOA obtained with contralateral stimulation normal subjects and in patients with retrocochlear disorder:
MATERIAL AND METHODOur material consisted of a group of subjects with nom hearing and another one with subjects who had retrocochlear disorders, treated between 1997 and 1998.
All subjects were informed about the study, its objectives, .procedures and exams to be performed; after that, each subject signed an authorization to participate. The study followed the ethical norms set forth by the Commission of Ethics at FMUSP, whose approval was obtained before the study actually started.
The first group consisted of 48 subjects, age range between 18 and 27 years, with hearing levels within normal range and no history of otological affections, divided into 46 female and 2 male patients.
The second group consisted of 9 subjects, age range between 33 and 76 years, divided into 4 women and 5 men. All of them had retrocochlear affections located at the level of the auditory nerve or brainstem, up to the superior olivary complex level. These subjects had been previously submitted to clinical neurological exam and imagining exams, either CT Scan or MRI.
All subjects who took part in this study were submitte to transient and distortion product EOA, with and without contralateral stimulation, in the following order:
o transient EOA with linear or nonlinear clicks, without contralateral stimulation.
o distortion product of EOA without contralateral stimulation,
o transient EOA with linear and nonlinear clicks, according to the first stimulation, using contralateral stimuli.
o distortion product of EOA, with contralateral stimulation.
To record the otoacoustic emissions, we used system Celesta 503 - Cochlear Emissions Analyzer (Version 3.xx), manufactured by Madsen Electronics.
Narrow band suppressor noise, presented contralaterally, was generated through a Madsen Electronics audiometer, model OB40, with sound filters, enabling presentation of noise at more effective intensities and at determined frequencies of 125 Hz to 500 Hz; from 750 Hz to 3.000 Hz; from 4.000 Hz to 8.000 Hz. Therefore, when conducting the exam for detection of suppression of distortion product otoacoustic emissions noise was presented in the most specific ranges of tested frequencies.
Upon the beginning of the exam, we consistently checked the permeability of the probe in order to avoid preexisting blocks, such as the presence of cerumen, which would result in altered results. Therefore, we assured that the external ear canal was always clean and free, avoiding the production of artifacts. In addition, we took extra caution because the probe contains a delicate transducer, and it should not be folded, put in contact with liquids or damaged in any way.
Before each phase was started, we checked the adjustment of the probe in the external ear canal to maintain conditions of stability - a resource available in the piece of equipment we used. This procedure helped us optimize the position of the probe in the external ear canal of each subject, resulting in reliable and reproducible responses.
In order to record transient otoacoustic emissions we used nonlinear clicks as stimuli - three clicks of one polarity and one click of inverse polarity, with a three-fold higher amplitude than the 15th click, of 100m/s duration, 70 to 80 dBSPL intensity in a total number of 3,000 accepted stimuli.
Presence or not of suppression was noticed with both linear and nonlinear clicks. Frequencies covered were between 500Hz and 4.000Hz. Clicks presented as stimuli were condensed.
To record distortion product, we used pure tones of different frequencies, in the ratio f2 /fl=1,22, for the region 750Hz, 1.000Hz, 2.000Hz, 3.000Hz, 4.000Hz, 6.000Hz and 8.000Hz. Pure tones at 70dBSPL were presented in a maximum total number of 1,000 stimuli; the number of stimuli was reduced when the record of otoacoustic emissions was significant. The record of these emissions was obtained through the ratio (2f1/f2).
Latet, these emissions were recorded again, but with a concomitant presentation of narrow band noise in the contralateral ear. The intensity of noise was about 5 to l0dB above the previous sound stimuli but below the level of stapedial reflex in the corresponding ear.
To study distortion product otoacoustic emissions, output of noise was obtained with more effective intensities, at specific frequency ranges of 125 Hz to 500 Hz; from 750 Hz to 3.000 Hz; from 4.000 Hz to 8.000 Hz.
Data obtained from the record of transient and distortion product otoacoustic emissions, both in normal hearing subjects and in patients with retrocochlear pathologies, were measured. We compared the amplitude values of emissions in dBSPL, especially concerning tested ear and tested frequencies and if they had been obtained with or without contralateral stimulation. The comparison among the tests with and without stimulation was conducted in order to define if there had been suppression of emissions.
Variables were presented by mean, standard deviation and median. Due to great variability of data and different sample sizes, we employed non-parametric tests.
RESULTSWe observed that suppressions obtained with contralateral stimulation were widely variable. However, most of the times, they were within the values of 1 to 3 dBSPL for transient emissions and 0.5 to 3dBSPL for distortion product.
We also observed, based on data presented in Graph 1, that in normal subjects there was suppression for transient otoacoustic emissions at all frequencies, whereas in the group with retrocochlear disease, there was an intensification of emissions at most frequencies.
In normal hearing subjects, the mean differences for all frequencies showed the presence of suppression for distortion product otoacoustic emissions (Graph 2).
In subjects with retrocochlear pathology, there was an intensification of emissions at frequencies 750 Hz, 1.000 Hz, 2.000 Hz and 3.000 Hz, and suppression at frequencies 4.000 Hz, 6.000 Hz and 8.000 Hz, for distortion product EOA (Graph 2).
When we compared the differences between the values of transient EOA measured in the normal subjects and in the patients with retrocochlear pathology, with and without contralateral stimulation, using narrow band noise, we observed the following characteristics:
a) with linear click, there was statistically significant difference for frequencies 1.000 Hz, 1.500 Hz, 2.000 Hz and 3.000 Hz, and marginally significant difference for frequency 4.000 Hz;
b) with nonlinear click; there were statistically significant differences for frequencies .2.000 Hz arid 3.000 Hz, and no significant difference for frequencies 1.000 Hz, 1.500 Hz and 4.000 Hz (Graph 1). I
Upon studying the variation of values of transient otoacoustic emissions obtained with and without contralateral stimulation and at different frequencies in the normal group we observed that there was statistically significant difference for frequencies 2.000Hz and 3.000Hz, and marginally significant difference for frequencies 1.000Hz, and no statistically significant difference for frequencies 1.500Hz and 4.000Hz (Graph 1).
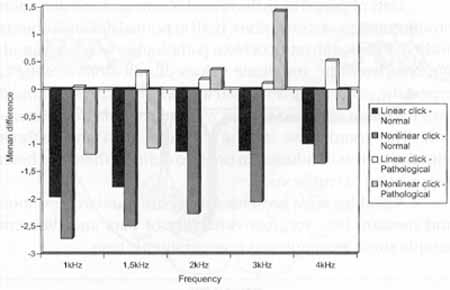
Graph 1. Comparison of the differences with and without contralateral stimulation found in the records of transient EOA in a group of normal hearing and retrocochlear affected subjects.
In the same study with transient otoacoustic emissions, when we analyzed patients with retrocochlear pathology, we observed a marginally significant difference for frequency 3.000 Hz and no statistically significant difference for frequencies 1.000 Hz, 1.500 Hz, 2.000 Hz and 4.000 Hz (Graph 1).
In the analysis of the differences of distortion product EOA comparing normal and retrocochlear patients, with or without contralateral stimulation and using narrow band noise, we observed statistically significant differences for frequencies 750 Hz, 1.000 Hz and 2.000 Hz; marginally significant differences for 3.000 Hz and no statistically significant differences for frequencies 4.000 Hz, 6.000 Hz and 8.000 Hz (Graph 2).
DISCUSSIONEvoked otoacoustic emissions are active mechanical responses that surge at the cochlea and provide information about the integrity of pre-synaptic cochlear receptor mechanisms20.
In addition, theoretically, it may be considered that normal otoacoustic emissions are obtained when auditory impairment is exclusively neural.
Apart from the clinical applications already demonstrated, currently there is also the possibility of a new use for otoacoustic emissions tests, considering the analysis of suppression as we present concomitant noise to the contralateral ear.
In our study, we tried to detect the normal characteristics of suppression of otoacoustic emissions using contralateral stimuli with narrow band noise.
We also tried to understand its formation mechanism, such as the participation of efferent auditory nervous system; in addition, we analyzed comparatively the used stimuli, linear and nonlinear clicks, pure tone of two close frequencies f1 and f2. On top of that, we also attempted to verify external situations that would interfere in the record of otoacoustic emissions and compare these findings with those found in patients with retrocochlear pathology in order to des possible clinical applications for this technique.
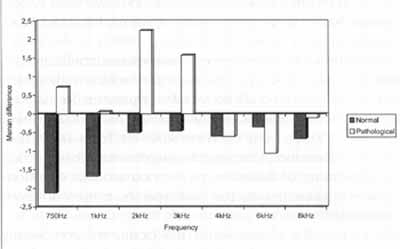
Graph 2. Comparison of the differences with and without contralateral stimulation found in the records of distortion product EOA in a group of normal hearing and retrocochlear affected subjects.
Despite the fact that the olivocochlear system initially described many decades ago14, 28, its study has be more emphasized in recent years. Physiology of medial efferent olivocochlear system involves interaction-of contralateral sounds in ipsilateral responses evoked by sound stimuli. This fact would be due to the existence of binaural excitable neurons, which have bilateral cochlear representation. This stimulation causes alterations in the resistance membranes of outer hair cells, modulating their potentials5, 17. Thus, this is the possibility of suppressing the emissions, be by ipsilateral and contralateral stimulation22. Despite the existence of this well documented evidence in the literature, doubts concerning the functioning of efferent auditory system are still very significant29.
We used contralateral stimulation to study the possible activity of medial efferent system and the effects produced otoacoustic emissions, following the model of other authors2, 3, 9.
In our study, we obtained suppression of transient otoacoustic emissions both originated from linear and nonlinear clicks. It would mean a reduction in the movement of outer ha cells, as a result of the increase in conductance of cells, an consequent hyperpolarization, leading to reduction of movement of basilar membrane, as observed by other authors10. This efferent activity would be a result of the mediation of net rotransmitters existing in the efferent endings at the base of outer hair cells, especially acetylcholine12, 21.
Differences found in amplitudes of emissions, with or without contralateral stimulation, were widely variable, even in normal subjects, ranging from suppression equivalent to 7dBSPL to an increase of 6.5dBSPL for transient otoacoustic emissions. For distortion product otoacoustic emissions, values varied from suppression of 13dBSPL to an increase of 11dBSPL. However, in most of the times, suppression was within the values of 2 to 3 dBSPL for transient otoacoustic emissions at frequencies ranges of 2.000Hz; less than 1 dBSPL for frequencies higher than 2.000Hz, and between 1 and 3dBSPL for frequencies 750Hz and 1.000Hz, for distortion product.
Responses were considered consistent when reproducibility rate of responses was above 70%.
In normal hearing subjects, the mean differences for all frequencies showed us the presence of suppression for transient otoacoustic emissions. In subjects with retrocochlear pathology, there was intensification of emissions at most frequencies.
We observed that contralateral stimulation, probably through efferent stimulation, reduces distortion product otoacoustic emissions, but sometimes also increases it, what is compatible with the data in the literature26. This inhibition is higher for low frequencies and it reduces as the frequency becomes higher25.
We observed a clear suppression of transient otoacoustic emissions using contralateral stimulation and narrow band noise. Checking of suppression was clearer when we used a linear click stimulus than with nonlinear clicks. With linear click, we observed a statistically significant suppression of recorded emissions, practically for all frequencies. Using nonlinear clicks, significant suppression was more evident for frequencies 2.000Hz and 3.000Hz, as already reported by other authors.
Record of transient EOA with nonlinear click is useful if we want to know whether the cochlear function is normal in regular clinical applications, in order to reduce artifacts of stimuli and exclude the participation of the middle ear - which is linear in its performance, and at the same time, record emissions in quantifiable manner. However, the use of sets of nonlinear clicks affects the true amplitude of emissions, transforming the absolute quantification of the suppression into a difficult task. Therefore, .having in mind the study of suppression, the use of linear clicks is both possible and useful, because the measure taken is in fact the difference between otoacoustic emissions obtained with and without contralateral stimulation, as confirmed by different authors1, 19. They started to conduct suppression tests with nonlinear clicks, and later they adopted linear clicks as a more practical option. Since suppression values are usually very small, tests with linear clicks make visualization of suppression a lot easier.
When comparing the suppression existent in transient otoacoustic emissions originated from linear clicks in normal subjects and in patients with retrocochlear pathology, we noticed that the difference was significant for frequencies 1.000Hz and 3.000Hz; when the click was nonlinear, differences were significant for frequencies 2.000Hz and 3.000Hz. In another study, it is shown that the effect of suppression is not hearing loss-dependent9. Nevertheless, hearing losses above 4.000Hz are not important, because transient otoacoustic emissions measured above 4.000Hz are very small because the frequency range of the click is between 1.000Hz and 4.000Hz.
We employed in the study suppression with contralateral stimulation narrow band noise because we believe that it is more effective in the process of suppression, since it has frequency selectivity, as shown in the comparative study using narrow band and wide band noise and pure tone1.
In our study, we noticed that distortion product otoacoustic emissions were affected by the suppression, when using contralateral noise, but suppression was clearer for frequencies 7~0 Hz, 1.000 Hz and 2.000 Hz, and they were practically absent in regions of higher frequencies, such as 4.000Hz, 6.000Hz and 8.000Hz. For distortion product otoacoustic emissions, mean absolute values of suppression were between 1 and 3dB for frequencies 750Hz, 1.000Hz and 2.000Hz. Suppression for distortion product also had wide variations in tests conducted with normal hearing subjects.
CONCLUSIONIn our study we detected the constant suppressive effect of otoacoustic emissions caused by contralateral sound stimulation, both for transient and distortion product in subjects with normal hearing. On the other hand, when we tested patients with retrocochlear pathologies, otoacoustic emissions could present suppression in some situations, be maintained the same in others, and also intensify some amplitudes.
Based on that, we suggested that recording of otoacoustic emissions with contralateral stimulation be considered useful, together with other procedures, as an effective method for diagnosis of retrocochlear diseases.
However, the effect of suppression was minor, suggesting the need for methodology and test protocols in order to be reliable and repeated if the interest is in using it as a clinical tool to help diagnosis.
Currently, cochlear origin of otoacoustic emissions is well documented and it is normally accepted. However, many issues remain unanswered and only a comprehensive understanding will provide the necessary answers. In addition, it is also important to learn about neural control.
Contralateral stimulation may be considered a support to evaluate the presence and the values of otoacoustic emissions as a probable method of study of the effects of efferent auditory system, especially of the medial bundle.
Efferent auditory nervous system may play an important role in the cochlear physiology and it surely requires further investigation.
REFERENCES1. BERLIN, C. I.; HOOD, L. J.; CECOLA, R. P.; JACKSON, D. F. e SZABO, P. - Does type I afferent neuro-dysfunction reveal itself through lack of efferent suppression? Hear. Res, 65, p. 4050, 1993.
2. BERLIN, C. I.; HOOD, L. J.; HURLEY, A. E.; WEN, H.; KEMP; D. T. -Binaural noise suppresses linear click-evoked otoacoustic emissions more than ipsilateral or contralateral noise. Hear. Res., 87, p. 96-103, 1995.
3. BERLIN, C. I. -Advanced concepts in ABR and otoacoustic emissions. In: ANNUAL MEETING OF NORTH AMERICAN ACADEMY OF OTOLARYNGOLOGY - HEAD AND NECK SURGERY, 100th, Washington, 1996. Abstracts. Washington, 1996. p.1-24.
4. BOBBIN, R. P. - Chemical receptors on outer hair cells and their molecular mechanisms. In: BERLIN, C.I., ed. Hair Cells and Hearing Aids. San Diego, Singular Publishing Group, Inc., 1996. p. 29-55.
5. BROWN, M. C. - Morphology and response properties of single olivocochlear efferent in the guinea pig. Hear. Res.; 40, p. 93-110, 1989.
6. BROWNELL, W. E. - Cochlear transduction: an integrative model and review. Hear. Res., 6, p. 335-60, 1982.
7. BROWNELL, W. E. - Observations on a motile response in isolated outer hair cells. In: WEBSTER, W. R.; AITKEN, L. M., eds. Mechanisms of hearing. Monash University Press, 1983. p. 5-10.
8. BROWNELL, W. E.; BADER, C. R.; BERTRAND, D.; RIBAUPIERRE, Y. - Evoked mechanical response of isolated cochlear outer hair cells. Science, 227, p. 194-6, 1985.
9. COLLET, L.; KEMP, D. T. E.; VEUILLET, E.; DUCLAUX, R.; MOULIN, A. E. MORGON, A. - Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear. Res., 43, p. 251- 62, 1990.
10. DALLOS, P. - Outer hair cells: the inside story. Am. Otol. Rhinol. Laryngol., 106, p. 16-22, 1997.
11. DOLAN, D. F.; NUTTAL, A. L.- Basilar membrane movement evoked by sound is altered by electrical stimulation of crossed olivocochlear bundle. Assoc. Res. Otolaryngol., Abstr., p.1789, 1994.
12. EYBALLIN, M. -Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol. Ver., 73, p. 309-73, 1993.
13. FLOCK, A.; FLOCK, B.; UNFENDAHL, M. -Mechanisms of movement in outer hair cells and a possible structural basis. Arch. Otorhinolaryngol., 243, p. 83-90, 1986.
14. GALAMBOS, R. - Suppression of auditory activity by stimulation of efferent fibers to the cochlea) Neurophysiol., 19, p. 424-37, 1956.
15. GATTAZ, G.; CERRUTI, V. Q. - O use do registro de emissões otoacusticas evocadas para triagem auditiva em neonatos de risco para deficiência auditiva. Rev. Paulista de Pediatria., 12, p. 291-4, 1994.
16. GOLD, T. -The physical basis of the action of the cochlea. Proc. R. Soc. Lond. Biol. Sci., 135, p. 492-8, 1948 APLID BROWNELL, W.E. Outer hair cell electromotility and otoacou stir emissions. In: BERLIN, C.I., ed. Hair Cells and Hearing Aids, San Diego, Singular Publishing Group, Inc., 1996. p. 3-27.
17. GUINAN, J. J. JR. - Physiology of olivocochlear efferents. In: DALLOS, P.; POPPER, AX; FAY, R. R. eds. The cochlea. New York, Springer-Verlag Inc., 1996. p. 435 - 502.
18. GUINAN, J. J. JR.; WARR, W. B.; NORRIS, B. E. -Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J. Comp. Neurol., 221, p. 358 70, 1983.
19. HOOD, L. J.; BERLIN, C. I.; HURLEY, A.; WEN, H. - Suppression of Otoacoustic Emissions in normal hearing individuals. In BERLIN, C.I., ed. Hair Cells and Hearing Aids. Singular Publishin Group, Inc., San Diego, 1996. p. 57-72.
20. KEMP, D. T.-Stimulated acoustic emissions from the human auditory system. J. Acoust. Soc. Am.; v. 64, 1386 -91, 1978.
21. KURC, M.; DODANE, V.; PINTO, D. S.; KACHAR, B. Presynaptic localization of G protein isoforms in the efferent nerve terminals of the mammalian cochlea. Hear. Res., 116, p. 1-9, 1998.
22. LIBERMAN, M. C. - Rapid assessment of sound-evoked olivocochlear feed-back: suppression of compound action potentials by contralateral sound. Hear. Res., 38, p. 47-56, 1989.
23. LONSBURY-MARTIN, B. L; MARTIN, G. K.; MCCOY, M. J.; WHITEHEAD, M. L. - New approaches to the evaluation of the auditory system and a current analysis of otoacoustic emissions. Otolaryng. - Head Neck Surg., 112, p. 50-63,1995. Supplement 1.
24. LOPES FILHO, O.; CARLOS, R. C.; ROSSI, H. J. Z.; ECKLEY, C. A.; GALLACI, C. B. -Emissoes otoacusticas espontaneas em recém-nascidos de risco. Revista Brasileira de Otorrinolaringologia, 63, p. 567-73, 1997.
25. MOULIN, A.; COLLJT, L.; DUCLAUX, R.- Contralateral auditory stimulation alters acoustic distortion products in humans. Hear. Res.; 65, p. 193-210, 1993.
26. MOUNTAIN, D. C.; GEISLER, C. D:; HUBBAND, A. E. Stimulation of efferent alters the cochlear microphony and the sound induced resistance changes measured in scala media of the guinea pig. Hear. Res., 3, p. 231-40, 1980.
27. PUEL, J. L.; REBBILARD, G. - Effect of contralateral sound stimulation on the distortion product 2 F1-F2. Evidence that the medial efferent system is involved. J. Acoust. Soc. Am., 87, p. 1630-5, 1990.
28. RASMUSSEN, G. L. - The olivary peduncle and other fiber projections of the superior olivary complex. J. Comp. Neurol., 84, p. 141-219, 1946.
29. WARR, W. B. -Organization of olivocochlear efferent systems in mammals. In: WEBSTER, D.B.; POPPER, A N.; FAY, R.R. eds. The mammalian auditory pathways. Neuroanatomy. New York Spring Verlag Inc., 1992. p. 410-48.
30. WARR, W. B.; GUINANJ. J. JR.; WHITE, J. S. -Organization of the efferent fibers: the lateral and medial olivocochlear systems In: ALTSCHULER, R. A.; HOFFMAN, D. W.; BOBBIN, R. P. eds Neurobiology of hearing: The Cochlea. New York, Raven Press 1986. p. 333-48.
* Ph.D. Professor of Pontifícia Universidade Católica de São Paulo.
** Ph.D. Professor of the Department of Otorhinolaryngology at Faculdade de Medicina da Universidade de São Paulo.
*** Ph.D. Professor of Pontifícia Universidade Católica de São Paulo.
Partial publication of the Doctorate Dissertation thesis presented to the Discipline of otorhinolaryngology at Faculdade de Medicina da Universidade de São Paulo in 1999.
Study conducted at Instituto de Otorrinolaringologia de São Paulo (Department of Otorhinolaryngology at Hospital dos Defeitos da Face).
Address for correspondence: Avenida Adolfo Pinheiro, 2464 - Conj 82 - Alto da Boa Vista - 04734-004 São Paulo/ SP - Tel/Fax: (55 11) 246-2581.
Article submitted on April 5, 2000. Article accepted on September 14, 2000.


