INTRODUCTIONSinonasal inverted papillomas are benign tumors that represent 0.5 to 4% of all nasal tumors3, 10, 19. They are more frequent in male individuals (5:1)1, 3, 7, 17, in Caucasians and between the 5th and 61th decades of life14, 18.
It was first described by Ward, in 1854; and it was characterized as a tumor by Ringertz in 193817.
Although there are some divergences concerning its etiology, malignancy process and surgical treatment, inverted papillomas show very defined characteristics: they are predominantly unilateral, are prone to recurrence and also have destructive capacity.
It differs fundamentally from other types of nasal papillomas because of its clinical and histopathological behavior. They are smooth polypoid masses that grow by the inversion of the epithelium inside the stroma of connective tissue15, 17 (Figures 1 and 2).
Etiology of inverted papilloma is unknown. Its association with viral infections has been demonstrated, especially concerning the relation with HPV and malignization of inverted papilloma6, 12, 14, 17, 18. Occupational factors such as exposure to fumes, dust and aerosol are also considered suspects in its pathogenesis4.
Its most frequent anatomical location is at the level of the lateral wall of the nose and the middle meatus, and ethmoid and maxillary sinuses are the most affected ones2, 9, 13, 16, 17 - although in more advanced stages, there may be orbital and intracranial invasions.
Clinical staging of inverted papilloma is done by similarity with olfactory neuroblastoma, according to Kadish11:
Stage A: confined to nasal fossa;
Stage B: confined to nasal fossa and paranasal sinuses;
Stage C: goes beyond the paranasal sinuses, towards the orbit and/or soft tissues;
Stage D: goes beyond paranasal sinuses, towards nasopharynx and/or intracranial area.
Clinically, it is manifested with unilateral nasal obstruction, rhinorrhea, pain or facial pressure and they frequently have a history of previous surgeries (polypectomy, ethmoidectomy, Caldwell-Luc)17. Surgical treatment is indicated and varies according to extension of lesion.
Radical surgery with medial maxillectomy in "monoblock" is the procedure that has presented the best results, however, endoscopic surgery has also been used in well delimited cases without compromise of paranasal sinuses9, 13.
This study focus diagnosis and management in 30 cases of sinonasal inverted papilloma treated in the period between 1985 and 1998.
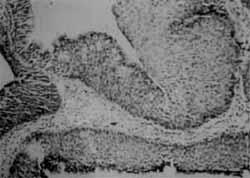

Figures 1 and 2. Histologic section of inverted papilloma showing the inversion of the epithelium inside-the adjacent stroma (HE, 10x).
We reviewed the medical files of 30 patients with sinonasal inverted papilloma treated between 1985 and 1998 at Nucleo de Otorrinolaringologia a Cirurgia de Cabeça e Pescoço de Sao Paulo. Twenty-seven patients had only inverted papilloma and 3 had inverted papilloma associated with squamous cell carcinoma. Assessment of patients was conducted through complete ENT exam, nasal videoendoscopy and CT scan. In 18 cases, pre-op biopsies were performed and confirmed the diagnosis of sinonasal inverted papilloma. In 6 cases there was suspicion of associated malignancy, and pre-op frozen biopsy was performed and sinonasal inverted papilloma was confirmed, and associated malignancy was detected in 2 cases. One case had the confirmation of associated malignancy only after the postoperative anatomical pathologic analysis. Six operated cases had a confirmation of sinonasal inverted papilloma based on the anatomical pathologic analysis of the surgical piece. We also collected data on age, sex, race, clinical picture, location of lesions, associated malignancy and treatment of inverted papilloma in our service. The mean follow-up was 36 months (12 to 132 months).
RESULTSAge, sex and race
Mean age was 51 years, with peak of incidence between the 5th and 6th decades of life. The predominance was in male subjects, in the proportion of 5 men to 1 woman. Racial proportion was 25 Caucasian patients (83.3%) to five Black patients (16.7%).
Signs and symptoms
The most common symptomatology was nasal obstruction, unilateral in 29 cases (96.6%), and bilateral in one case (3.4%).Other studied symptoms, all associated with nasal obstruction, were: epistaxis in 12 cases (40%), headache in 13 cases (43.3%), mucous-purulent rhinorrhea in 25 cases (83.3%), facial pain in 5 cases (16.6%), orbital compromise in one case (3.3%) and nasal deformity in 2 cases (6.6%).
The exam of nasal fossae revealed the presence of a mass, with polypoid characteristics. Eighteen cases (60%) had history of previous surgeries.
Location of lesions
There were 30 cases (100%) that presented compromise of one or both nasal fossae, and one or more sinuses could also be affected; isolate affection of nasal cavity was found in 3 cases (10%). The most affected paranasal sinuses in decreasing order were: maxillary sinus, 27 cases (90%), ethmoid sinus, 17 cases (56.6%), sphenoid sinus, one case (3.3%), and frontal sinus was not affected in any case.
Clinical staging of sinonasal inverted papilloma in our series, according to Kadish classification, were 25 cases staged as B (83.4%), 3 as A (10%) and 2 as C (6.6%).
Associated malignancy
Three cases (10%) presented associated malignant squamous cell tumors detected in the histological exam.
Treatment
Surgical treatment was the preferred choice and the approach depended on staging. Sublabial via with middle facial degloving was used in 26 (86.6%) surgical procedures, with medial maxillectomy, and there was no recurrence in this group.
Endoscopic/microsurgical resection was conducted in 4 cases (13.3%). There was recurrence in 2 cases (6.6%) of patients submitted to endoscopic surgery, who later were reoperated via microsurgery with medial maxillectomy.
DISCUSSIONSinonasal inverted papilloma amounts to 0.5% to 4% of all sinonasal tumors3, 10, 19. Due to its low incidence, epidemiological study of this tumor requires long term data. In our series, we gathered 30 cases in a 13-year period, corresponding to 2.3 new cases per year. Vrabec (1994)19 found 101 cases in a 25year period (4 cases/year) and Lawson et al. (1995) 10 found 112 cases in 20 years (5.6 cases/year).
The predominance of male individuals in the proportion of 5 men to 1 woman is similar to that reported by the literature1, 3, 7, 17. As to age of patients at the time of diagnosis, we noticed that it ranged from 25 to 70 years, and the incidence was higher between the 5th and 6th decades of life, in accordance with the data by Myers (1981)15 and Vrabec (1994)19.
According to Ringertz's description in 1938, inverted papilloma is histologically characterized by growth and invasion of epithelium inside the stroma of connective tissue, and it is prone to recur if resection is not complete17. It is compatible with the description by Olphen, 1988, and Ingle, 19988, 16.
The cause of the tumor is still unknown. Recent studies suggested that papillomavirus (HPV) played an important role in its pathogenesis, and some types of virus may be associated with its malignant transformation6, 14, 17, 18. An increase in incidence of this pathology in patients exposed to varied fumes, dust and aerosol was shown, but we still lack more studies to confirm this possibility4.
Most frequent anatomical location of the lesion is at the level of the lateral nasal wall and middle meatus, and it is important to point out that after the nasal fossae, ethmoid and maxillary sinuses were the most compromised areas2, 9, 13, 16, 17. In our series, maxillary and ethmoid sinuses, together with the nasal fossae, were also the most affected sites (83.3%) (Figures 3 and 4). The location is different from that of exophytic papillomas, which are located multicentrally in the nasal septum2. Invasion of orbit and peri-orbital soft tissues may occur, at more advanced stages, in which destruction of papyracea lamina is present, with excessive growth of tumor. In 1995 Elner described 10 cases with orbital invasion5.
Nasal obstruction (100%), mucous-purulent rhinorrhea (83.3%), headache (43.3%), epistaxis (40%), facial pain (16.6%), nasal deformity (6.6%) and orbital alterations (3.3%) represented the initial complaints of patients, also in agreement with the description in the literature2, 15, 17, 18, emphasizing the importance of having differential diagnosis between sinonasal chronic infection and sinonasal tumors12. We believe that owing to the similarities in the clinical presentation of nasal infectious and tumors, the hypothesis of tumor should always be considered, contributing to early diagnosis.
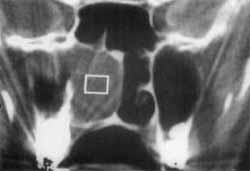
Figure 3. CT scan showing tumor mass in the right nasal fossa and right maxillary sinus diagnosed as inverted papilloma (stage B). Preoperative image.
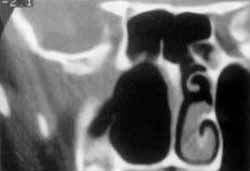
Figure 4. CT scan showing postoperative aspect of right redial maxillectomy, with removal of tumor mass.
At rhinoscopy, sometimes it is difficult to distinguish inflammatory nasal polyps from inverted papilloma. Many times both are present, but the difference is that polyps are normally bilateral and inverted papilloma is unilateral. If there is suspicion of inverted papilloma, a biopsy with local anesthesia should be made before the definite treatment.
In our study, there was recurrence in two cases submitted to resection with conservative endoscopic surgery and they had to undergo a new procedure of medial maxillectomy with microsurgery. There are descriptions such as the one by Tosz, in 1995, in which recurrence was 50% in 28 patients submitted to resection, via lateral rhinotomy, with medial maxillectomy. Recurrence of exophylic papilloma is very common and it is found in up to 66% of the cases, according to reports of the author2. Recurrence in cases of orbital invasion was 80%, according to Elner5.
One of the reasons attributed to tumor recurrence is the finding of metaplasic respiratory mucosa adjacent to tumor site. Therefore, if all visible tumor is resected, adjacent metaplasic mucosa may eventually progress into a tumor. Multicentric tumors are also found.
In 10% of our cases, there was association with squamous cell carcinoma diagnosed through anatomical pathologic exam after resection of tumor. In 3 cases, surgical treatment was considered complete, with maligning area removed as part of the resected monoblock. This was also similar to the data presented by other authors, such as Bernauer (13%)1 and Ravah (7%)18, whereas Lesperance found 14 cases associated with malignancy out of 51 studied cases, totaling 27%11. The difference is very significant for cases with orbital invasion; Elner described the association with squamous cell carcinoma in 100% of the 10 studied cases5.
Axial and coronal CT Scan is important to assess the extension of the disease, because the presence of concomitant retained secretion and tumor extension, magnetic resonance may be needed.
Advocated treatment in our service was surgical, via sublabial approach, with middle facial degloving and medial maxillectomy in 86.6% of the performed procedures, and endoscopic/microsurgical resection in 13.3% of the cases. Results were satisfactory, with recurrence in 2 cases (6.6%) submitted to endoscopic resection, re-operated via microsurgical medial maxillectomy. Many authors advocate the use of lateral rhinotomy with medial maxillectomy as the preferred approach, and they reached a cure rate similar to ours9, 17.
Transnasal approach is indicated for tumors limited to the septum or inferior and middle turbinate, which should be completely resected, together with the study of surgical margins.
We considered the incidence of 10% quite high for association of sinonasal inverted papilloma and squamous cell carcinoma, as well as its recurrence. Therefore, agreeing with Lesperance, we advocate long term follow-up, using clinical exam, nasal videoendoscopy and CT scan in all patients with inverted papilloma, especially those submitted to resection through endoscopic surgery11.
CONCLUSIONSinonasal inverted papilloma is a relatively infrequent nasal tumor.
Due to non-specific symptoms, we should perform differential diagnosis with other tumors and sinonasal chronic infectious conditions.
Although today there are results with more limited approaches, in our opinion, high recurrence is observed by some authors because of incomplete resection, not done in monoblock.
Since most of the tumors are diagnosed at stage B, medial maxillectomy through sublabial approach provides better conditions to resect the tumor, reducing significantly recurrence rates.
Long term follow-up, including clinical exam, nasal videoendoscopy and CT scan should be conducted because of the likelihood of recurrence and association with squamous cell carcinoma.
REFERENCES1. BERNAUER, H. S.; WELKOBORSKYE, H. J.; TILLING, A. et. al. - Inverted Papillomas of the Paranasal Sinuses and the Nasal Cavity: DNA Indices and HPV Infection. American journal of Rhinology, 11: 155-160, 1997.
2. BUCHWALD, C.; TOS, M.; FRANZMANN, M. B. - Sinonasal Papillomas: A Report of 82 Cases in Copenhagen County, Including a Longitudinal Epidemiological and Clinical Study. Laryngoscope, 105 72 - 79, 1995.
3. DAMMANN, F.; PEREIRA, P.; LANIADO, M. et. al.- Inverted Papilloma of the Nasal Cavity and the Paranasal Sinuses: Using CT for Primary Diagnosis and Follow-up. AJR, 172: 543-548, 1999.
4. DEITMER, T.; WIENER, C.-Is there an Occupational Etiology of Inverted Papilloma of the Nose and Sinuses? Acta Otolaryngol, 116: 762 - 765, 1996.
5. ELNER, V. M.; BURNSTINE, M. A.; GOODMAN, M. L. -Inverted Papillomas That Invade the Orbit. Arch Ophthalomol, 103:1178 - 1183, 1995.
6. GAFFEY, M. J.; FRIERSON, H: F.;' WEISS, L. M. - Human Papillomavirus and Epstein-Bar Virus in Sinonasal Schneiderian Papillomas. An In Situ Hybridization and Polymerase Chain Reaction Study. A. J. C. P., 106 475-482, 1996.
7. GUICHARD, C.; GILAIN, L.; SAMAD, I. A. A. -Epithelial Cell Proliferation, Apoptosis, and Apoptosis Inhibition in Inverted Papillomas. Laryngoscope, 108: 716 - 720, 1998.
8. INGLE, R.; JENNINGS, T. A.; GOODMAN, M. L.; et. al. -CD44 Expression in Sinusal Inverted Papillomas and Associated Squamous Cell Carcinoma. A.J. C. P., 109: 309 - 314, 1998.
9. KAMEL, R. H. - Transnasal Endoscopic Medical Maxillectomy in Inverted Papilloma. Laryngoscope, 105: 847 - 853, 1995.
10. LAWSON, W.; HO, B. T.; SHAARI, C. M. et al. - Inverted Papilloma: A Report of 112 Cases. Laryngoscope, 105: 282-288, 1995.
11. LESPERANCE, M. M.; ESCLAMADO, R. M. -Squamous Cell Carcinoma Arising in Inverted Papilloma. Laryngoscope, 105: 178 - 183, 1995.
12. LUND, V. J. - Malignant Tumors of the Nasal Cavity and Paranasal Sinuses. ORL, 45:1-12, 1983.
13. MACCARRY, W. S.; GROSS, C. W.; REIBEL, J. F.-Preliminary Report: Endoscopic Versus External Surgery in the Management of Inverting Papilloma. Laryngoscope, 104: 415 - 419, 1994.
14. MACDONALD, M. R.; LE, K.T.; FREEMAN, J. -A Majority of Inverted Sinonasal Papillomas Carries Epstein-Barr Virus Genomes. Cancer, 75: 2307 - 2312, 1995.
15. MYERS, E. N.; SCHRAMM, V. L.; BARNES, E. L. -Management of Inverted Papilloma of the Nose and Paranasal Sinuses. Laryngoscope, 91: 2071 - 2084, 1981.
16. OLPHEN, A. F. V.; LUBSEN, H.; VERLAATJ. W. V.-An Inverted Papilloma With Intracranial Extension. The Journal of Laryngology and Otology, 102: 534 - 538, 1998.
17. PHILLIPS, P. P.; GUSTAFSON, R. O.; PACER, G. W. -The Clinical Behavior of Inverting Papilloma of the Nose and Paranasal Sinuses: Report of 112 Cases and Review of the Literature. Laryngoscope, 100: 463-469,1990.
18. RAVEH, E.; FEINMESSER, R.; SHPITZER, T. et. al. -Inverted Papilloma of the Nose and Paranasal Sinuses: A Study of 56 Cases and Review of the Literature. Israel J. Med. Sci., 32: 1163 1167, 1996.
19. VRABEC, D. P. -The Inverted Schneiderian Papilloma: A 25 - Year Study. Laryngoscope, 104: 582 - 605, 1994.
* Resident Physician with Nucleo de Otorrinolaringologia de São Paulo.
** Assistant Physician with Nucleo de Otorrinolaringologia de São Paulo.
*** Director of Nucleo de Otorrinolaringologia de São Paulo.
Núcleo de Otorrinolaringologia de São Paulo.
Address for correspondence: Jose A. Pinto - Al. Nhambiquaras, 159 - Indianópolis - 04090-010 São Paulo/ SP.
Tel/Fax: (55 11) 5573-1970 - E-mail: ppcintra@dglnet.com.br
Article submitted on March 31, 2000. Article accepted on August 17, 2000.


