INTRODUCTIONHuman communication processes mainly through language and speech and it is through audition that man acquires and maintains them.
Hearing loss has been considered a disabling disease because of the role of hearing in human communication. It may be caused by different etiological factors, congenital or acquired, and have different degrees and variables involved.
One of the causes of acquired hearing loss is the use of ototoxic drugs. These drugs damage the sensorial structure of the inner cells and they may affect both the auditory and vestibular systems.
Many factors may influence the toxicity potential, such as agent, dose, duration, renal function, previous use of other drugs and individual susceptibility.
The most common cause of permanent hearing loss by ototoxic drugs has been observed with the use of aminoglycosides and platinum by-products, such as cisplatin9.
Cisplatin is an alkylating anti-neoplasic chemotherapic agent derived from inorganic salts of platinum, and it has been used with good results in bone tumors, such as osteosarcoma.
Osteosarcoma is a primary malignant bone neoplasia, most frequently found in children and adolescents. It may affect any bone or even soft parts, but it is more commonly found in the humerus, tibia or femur.
The effective treatment approach against this tumor has been the combined use of chemotherapy and surgery.
As we said before, the use of cisplatin against this tumors has shown effective anti-tumor activity in humans beings, but it has a high degree of toxicity, causing side effects such as renal and hearing disorders1, 26.
Taking into account the hearing disorder caused by its use, it is important to monitor hearing of patients' using ototoxic drugs, in order to determine progression of ototoxicity once it starts.
It is known that initially the hearing damage is caused at the base of cochlea, affecting high frequencies and it may progress into the apex, affecting middle and low frequencies29.
Therefore, monitoring of hearing thresholds, especially in high frequencies, is important during administration of the drug.
It is important to point out that an effective program of monitoring of the effects of the drug on hearing would lead to early detection of hearing loss, enabling appropriate selection of alternative treatment approaches3, 11.
When thresholds alterations are noted, some measures may be taken by the specialist physician: interruption of medication, use of alternative treatments, reduction or change of dose, or carry on with the treatment but inform the family about a possible hearing loss24, 11, 21.
High frequency audiometry has been considered the preferred method for early detection of hearing loss induced by ototoxic drugs, because it enables detection of hearing alterations before key speech frequencies are affected9, 18, 14, 10, 12, 13. This procedure has proved to be more sensitive than otoacoustic emissions4, 5, because they record data up to 10,000Hz. We also know that there are audiometers that record frequencies up to 20,000Hz.
On the other hand, some authors mentioned that otoacoustic emissions could identify hearing abnormalities earlier than conventional pure tone audiometry, because modifications of amplitude of emissions may be noted before changes in hearing threshold25, 30, 22, 20, 17.
Experimental studies were started by Prof. Lazlo Stein (Personal Communication, 2000)28, at Northwestern University - Chicago - IL, using high frequencies to evoke otoacoustic emissions in order to monitor hearing of patients submitted to chemotherapy.
Agreeing with the fact that high frequency audiometry is an effective procedure for the assessment of ototoxic effects, American Speech-Language-Hearing Association (ASHA), in 19943, standardized a protocol recommending some procedures to monitor hearing of patients submitted to treatment with ototoxic drugs.
According to ASHA (1994)3, first an anamnesis should be performed, followed by otoscopy and later, the patient is submitted to an audiological assessment that consists of pure tone audiometry (conventional frequency range), high frequency audiometry (higher than 8,000Hz), speech audiometry and immitanciometry. Normally, speech audiometry and immitanciometry are conducted in the first session and they are only repeated if changes in hearing thresholds are noted.
If the patient is not able to .respond to subjective tests, objective measures may be employed, such as evoked brainstem potentials and otoacoustic emissions.
Based on these premises, the objective of our study was to monitor hearing thresholds of patients who had diagnosis of osteosarcoma and were submitted to chemotherapy with no previous treatment.
MATERIAL AND METHODThe material of Skis study consisted of hearing thresholds of 27 patients (54 ears) who had diagnosis of osteosarcoma, no previous treatment, and were submitted to chemotherapy with cisplatin, carboplatin and other associated drugs. Patients were referred from the Sector of Pediatric Oncology of the Department of Pediatrics at Universidade Federal de Sao Paulo -Escola Paulista de Medicina, and were assessed at the Ambulatory of the Discipline of Hearing Disorders at Universidade Federal de Sao Paulo - Escola Paulista de Medicina.
Out of 27 studied patients, 19 were male and 8 were female subjects, in the age range of 11 to 20 years.
All patients were submitted to anamnesis in order to collect information about the current pathology and previous events concerning hearing. Patients who had previous hearing complaints were excluded from the study.
Next, we performed otoscopy in order to investigate the presence of excessive cerumen, foreign bodies or any sign of affection that would hinder the conduction of audiological assessment.
Audiological assessment included conventional pure tone audiometry (250 to 8,000 Hz); high frequency audiometry (9,000 to 18,000 Hz); speech audiometry, comprising speech detection threshold and percentage index of speech recognition in sound-proof booth, and immitanciometry, including tympanometry and stapedial reflex, both contra and ipsilateral, in order to exclude middle ear affections.
For conventional pure tone audiometry and speech audiometry we used Maico audiometer, model MA-41, phones TDH-39 and MX-412. For high frequency audiometry, we used Interacoustics audiometer, model AS 10 HF and phones KOSS HV-1A, expressing hearing thresholds in dBSPL (Sound Pressure Level).
To analyze acoustic immitance, we used Interacoustics middle ear analyzes, model AZ-7.
All patients followed the same chemotherapic protocol consisting of 9 cycles of chemotherapy, with an interval of 21 days between cycles. During pre-op (week zero, 3 and 6), patients received 3 doses of 100 mg/m² of cisplatin (total of 300 mg/m²), 500 mg/m² of carboplatin (total of 1,500 mg/m²) and 70 mg/m² of doxorrubicin (total of 210 mg/m²). Post-operatively, two more doses were administered - 100 mg/m² of cisplatin (weeks 11 and 20, total of 200 mg/m²), two doses of 500 mg/m² of carboplatin (weeks 17 and 26, total of 1.000 mg/m²), three doses of 70 mg/m² of doxorrubicin (weeks 14, 17 and 23, total of 210 mg/m²) and 5 doses of 9 g/m² of iphosphamide/mesna (weeks 11, 14, 20, 23 and 26, total of 45 g/m²). As we may observe in the schematic table below, 100 mg/m² doses of cisplatin were administered in 5 out of 9 treatment cycles.
Schematic table of chemotherapy treatment:
S = Treatment week.
The total dose used in chemotherapy treatment was:
? Cisplatin 100 mg/m² X 5 = 500 mg/m²
? Carboplatin 500 mg/m² X 5 = 2,500 mg/m²
? Doxorrubicin 70 mg/m² X 6 = 420 mg/m²
? Iphosphamide 9 g/m² X 5 = 45 g/m²
? Mesna 9 g/m² X 5 = 45 g/m²
According to the protocol proposed, we performed 4 audiological assessments - one pre-treatment, two during it (weeks 6 and 17) and one after chemotherapy.
To study the thresholds, we used the same reference in all studied frequencies, and values obtained in dBHL (dB Hearing Level) - thresholds of frequencies 250 to 8,000Hz, were converted into dBSPL, according to values determined by ANSI-69².
Values proposed by Norm ANSI-69² for 0 dBHL:
For statistical analysis of results we used non parametric tests, taking into consideration the nature of variables studied. To check the differences between thresholds obtained for tested frequencies in the studied periods, for each frequency in each group, we used Friedman's non-parametric test for non-independent samples27, complemented by the test of multiple comparisons16.
In all tests, we determined 0.05 or 5% as the level to reject null hypothesis, and marked the significant values with an asterisk.
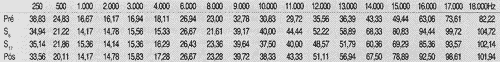
RESULTS AND DISCUSSIONFirst, we collected auditory thresholds for the 27 patients - 54 ears, in the frequency range 250 to 18,000Hz at pre, 6 and 17 weeks and post-treatment with chemotherapy, as seen in Tables 1, 2, 3 and 4.
We noticed that 9 patients -18 ears (33.3%) had normal auditory thresholds for conventional frequency range until the end of treatment; and 18 patients - 36 ears (66.6%) had altered auditory thresholds in conventional frequency range during chemotherapy. Therefore, the sample was divided as follows: Group 1 consisting of patients who had normal hearing thresholds - below 25dBHL, in conventional frequency range 250 to 8,000Hz after chemotherapy treatment; and Group 2, consisting of patients who had altered auditory thresholds in conventional frequency range after chemotherapy treatment (Table 5). These data show that hearing alterations may vary from patient to patient, because of individual susceptibility. This fact had already been reported by Aguilar-Markulis1 and Brock6.
Tables 6 and 7 show the mean auditory thresholds (dBSPL) obtained for each tested frequency (Hz) in pre, weeks 6 and 17, and post-treatment periods for Groups 1 and 2, respectively.
We observed a predominance of bilateral symmetrical sensorineural hearing loss, with predominance of high frequencies, both for Group 1 and Group 2, as shown in Tables 1 and 2. Bilateral symmetrical affection followed by high frequency loss was also described Aguilar-Markulis1 and Fausti9, 11.
TABLE 1 - Auditory thresholds (dB SPL) obtained in frequency ranges 250 to 18,000 Hz, according to tested ear in pre-chemotherapy treatment.
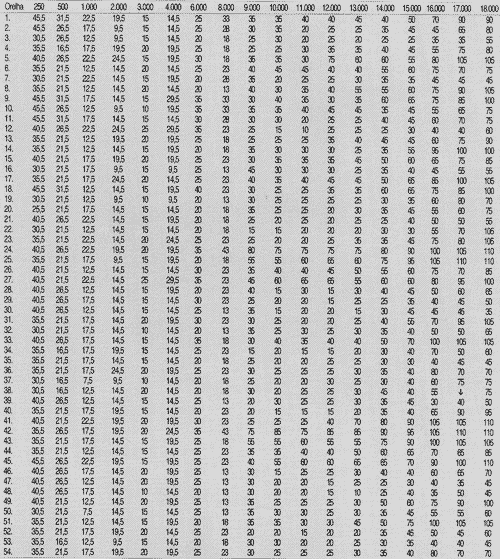
TABLE 2 - Auditory thresholds (dB SPL) obtained for frequency ranges 250 to 18,000 Hz, according to tested ear on week 6.
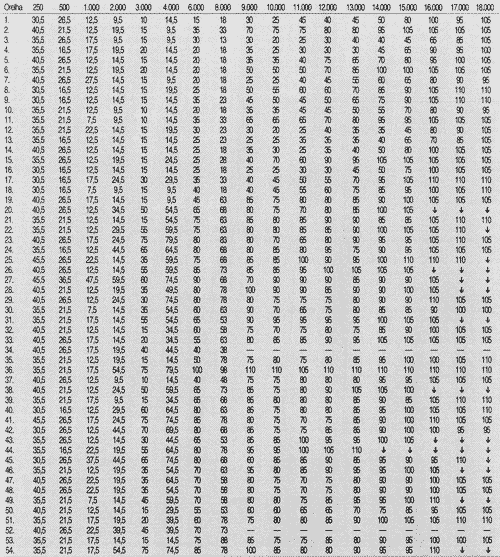
TABLE 3 - Auditory thresholds (dB SPL) obtained for frequency to tested ear on week 17.
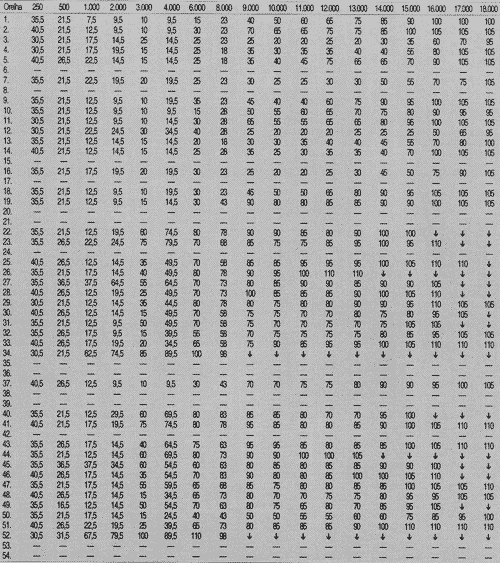
TABLE 4 - Auditory thresholds (dB SPL) obtained for frequency ranges 250 to 18,000 Hz, according to tested ear, in post-chemotherapic period.
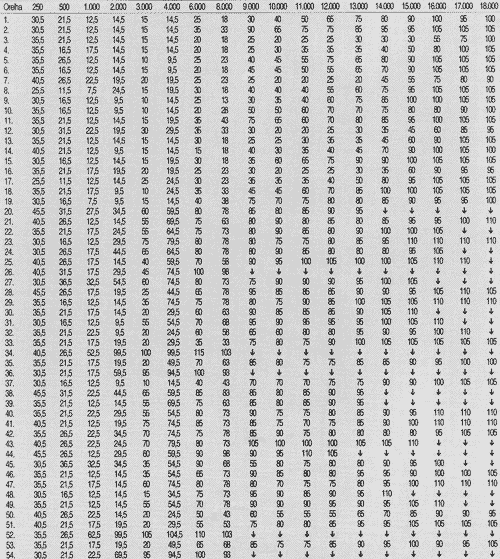
TABLE 5 - Subjects and ears according to conventional pure tone audiometry.

TABLE 6 - Mean values of auditory thresholds (dBSPL) obtained for frequency ranges 250 to 18,000 Hz, according to tested ear in Group 1, in periods pre, S6, S17, and post-treatment with chemotherapy.
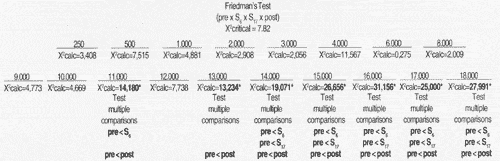
High frequency audiometry identified changes in auditory thresholds of 100% of the cases in the first assessment conducted during chemotherapy, at a dose of 300mg/m² of cisplatin. Many authors agree that there is a change in thresholds of high frequencies after administration of a high dose of cisplatin - higher than 300mg/m² 18, 19, 11, 23.
In Group 1, we observed that hearing alterations were present from the frequency of 11,000Hz on and there were no significant changes in auditory thresholds in the subsequent assessments. In Group 2, alterations were noted from frequency 2,000Hz, varying up to 70dB, and they were more marked between 3,000 and 18,000Hz. Park" did not observe significant changes of auditory thresholds in frequencies 250 to 2,000Hz, even after the total dose of cisplatin.
The onset of hearing affection started in week 6, that is, right after the administration of 300mg/m² of cisplatin, and we did not find statistically significant differences between thresholds obtained in week 6 and the remaining assessments. There was a trend towards stabilization of auditory thresholds even when the dose of cisplatin was increased, agreeing with the findings by Park23. This effect was called "plateau phenomenon": as from specific doses, there is no further worsening of hearing thresholds. This phenomenon was noticed by Skinner26 with a dose of 600mg/m² of cisplatin.
However, we did not perform audiological assessment of patients between the 3 first cycles of chemotherapy (S0, S3 e S6), in which the largest concentration of cisplatin was present. Owing to this fact, we did not define in which cycle the onset of hearing alterations took place. Other authors have reported more marked ototoxic effect at high doses, higher than 100mg/ m2 and elevated accumulative doses15, 29, 6.
TABLE 7 - Mean values of auditory thresholds (dBSPL), obtained for frequency ranges 250 to 18,000 Hz, according to tested ear, in Group 2, in pre, S6, S17 and post-treatment with chemotherapy.

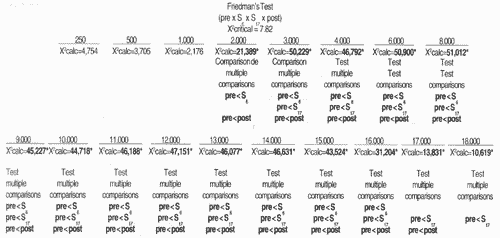
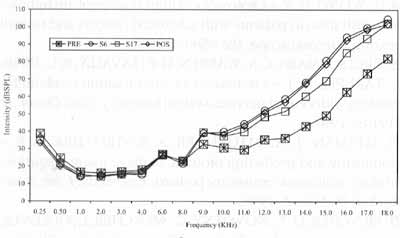
Figure 1. Mean values of auditory thresholds (dBSPL), obtained fox frequency ranges 0.25 to 18.0 KHz, according to tested ear, in Group 1, in periods pre, S6, Sl7 and post-treatment with chemotherapy.
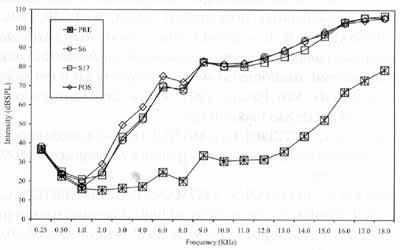
Figure 2. Mean values of auditory thresholds (dBSPL), obtained for frequency ranges 0.25 to 18.0 KHz, according to tested ear, in Group 2, in periods pre, S6, Sl7 and post-treatment with chemotherapy.
Chemotherapy treatment proposed by the present protocol results in bilateral symmetrical sensorineural hearing loss, predominantly in high frequencies.
In 33.3% of the cases, it induced hearing loss only in high frequencies.
In 66.6% of the cases, it caused hearing loss that started in conventional frequencies range.
With a 300mg/m² dose of cisplatin there are significant changes in auditory thresholds, maintained until the end of chemotherapy, and there were no significant changes in the subsequent assessments.
These data suggest that audiological assessment be conducted between the 3 first cycles of 100 mg/m² doses of cisplatin (S0, S3 a S6).
REFERENCES1. AGUILAR-MARKULIS, N. V.; BECKLEY, S.; PRIORE, R.; METTLIN, C. - Auditory toxicity effects of long-term cisdichlorodiammineplatinum II therapy in genitourinary cancer patients. J. Surg. Oncol., 16: 111-23, 1981.
2. AMERICAN NATIONAL STANDARD INSTITUTE -American National Standards Specification for audiometers. American Standard S3.6-1969. New York, 1969.
3. AMERICAN SPEECH-LANGUAGE-HEARING ASSOCIATION - Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA, 36 11-19, 1994.
4. BENSADON, R. L. - Estudo clinico da ototoxicidade da cisplatina: comparação entre os exames de audiometria tonal convencional, audiometria de altas freqüências a emissões otoacusticas. São Paulo, 1999. [Tese de Doutorado Universidade de São Paulo] 100p.
5. BERG, A. L.; SPITZER. J. B.; GARVIN. J. H. Jr. - Ototoxic impact of cisplatin in pediatric oncology patients. Laryngoscope, 109: 1806-14, 1999.
6. BROCK, P.; BELLMAN, S.; YEOMANS, E. C.; PINKERTON, C. R.; PRITCHARD, J. - Ototoxicity of high dose cisplatinum in children: a practical grading system. Med. Pediatr. Oncol., 19: 295-300, 1991.
7. DAVIS, H. & SILVERMAN, S. R. -Hearing and deafness. 3. ed., New York, Rinehart & Winston, 1970. 522p.
8. DISHTCHEKENIAN, A.; KECHICHIAN, R.; PETRILLI, A. S.; AZEVEDO, M. F.; IORIO, M. C. M. - A study of the hearing of osteosarcoma patients treated with carboplatin and cisplatin chemotherapy. In: CONGRESSO DA ACADEMIA AMERICANA DE AUDIOLOGIA, X. Los Angeles, 1998. Anais. Los Angeles, 1998.
9. FAUSTI, S. A.; SCHECHTER, M. A.; RAPPAPORT, B. Z.; FREY, R. H.; MASS, R. E. -Early detection of cisplatin ototoxicity. Cancer, 53: 224-31, 1984.
10. FAUSTI, S. A.; FREY, R. H.; HENRY, J. A.; OLSON, D. J.; SCHAFFER, H. 1. - High-frequency testing techniques and instrumentation for early detection of ototoxicity. J. Rehab. Res. Dev., 30(3): 333-41, 1993a.
11. FAUSTI, S. A.; HENRY, J. A.; SCHAFFER, H. I.; OLSON, D. J.; FREY, R. H.; BAGBY Jr., G. C. - High frequency monitoring for early detection of cisplatin ototoxicity. Arch. Otolaryngol. Head Neck Surg., 119: 661-6, 1993b.
12. FAUSTI, S. A.; LARSON, V. D., NOFFSINGER, D., WILSON, R. H., PHILLIPS, D. S.; FOWLER, C. G. - High-frequency audiometric monitoring strategies for early detection monitoring of ototoxicity. Ear Hear., 15: 232-39, 1994.
13. FAUSTI, S. A.; HENRY, J. A.; HAYDEN, D.; PHILLIPS, D. S.; e FREY, R. H. - Intrasubject reliability of high-frequency (9-14kHz) thresholds: tested separately vs. following conventional frequency testing. J. Am. Acad. Audiol., 9: 147-52, 1998.
14. FRANK, R. & DREISBACH, L: E. - Repeatability of high frequency thresholds. Ear Hear., 12: 294-95, 1991.
15. HALL, D. J.; DIASIO, R. & GOPLERUD, D. R. - Cis-platinum in gynecologic cancer. Am. J. Obstet. Gynecol., 141: 309-12, 1981.
16. HOLANDER, M. & WOLFE, D. A. -Nonparametric statistical methods. New York, John Wiley & Sons. 1973. 503p.
17. KASHIWAMURA, M. -Studies of the evaluation of cochlea function with distortion product otoacoustic emission. Hokkaido Igaku Zasshi, 73: 641-62, 1998.
18. KOPELMAN, J.; BUDNICK, A. S.; SESSIONS, R. B.; KRAMER, M. B.; WONG, G. Y. - Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope, 98: 858-64, 1988.
19. KRETSCHMARN, C. S.; WARREN, M. P.; LAVALLY, B. L.; DYER, S.; TARBELL, N. J. - Ototoxicity of pre-radiation cisplatin for children with central nervous system tumors . J. Clin. Oncol., 8: 1191-8, 1990.
20. LITTMAN, T. A.; MAGRUDER, A. & STROTHER, D. R. Monitoring and predicting ototoxic damage using distortion product otoacoustic emissions: pediatric case study. J. Am. Acad. Audiol, 9: 257-62, 1998.
21. MENCHER, G. T.; NOVOTNY, G.; MENCHER, L.; GULLIVER, M. - Ototoxicity and irradiation: additional etiologies of hearing loss in adults. J. Am. Acad. Audiol, 6: 351-57, 1995.
22. OZTURAN, O.; JERGER, J.; LEW, H.; LYNCH, G. R. Monitoring of cisplatin ototoxicity by distortion-product otoacoustic emissions. Auris Nasus Larynx, 23: 147-51, 1996.
23. PARK, K. R. - The utility of acoustic reflex thresholds and other conventional audiologic tests for monitoring cisplatin ototoxicity in the pediatric population. Ear Hear., 17: 107-15, 1996.
24. PASIC, T. R. & BOBIE, R. A. - Cis-platinum ototoxicity in children. Laryngoscope, 101: 985-91, 1991.
25. PROBST, R.; HARRIS, F. P. & HAUSER, R. -Clinical monitoring using otoacoustic emissions. Br. J. Audiol, 27- 85-90, 1993.
26. SKINNER, R.; PEARSON, A. D. J.; AMINEDDINE, H. A.; MATHIAS, D. B.; CRAFT, A. W. - Ototoxicity of cisplatinum in children and adolescents. Br. J. Cancer, 61: 927-30, 1990.
27. SIEGEL, S. - Estatística não parametrica. Mexico, Trillas. 1975. 346p.
28. STEIN, L. - Comunicação pessoal. North western University - Chicago - IL, 2000.
29. STUART, N. S. A.; WOODROFFE, C. M.; GRUNDY, R.; CULLEN, M. H. - Long-term toxicity of chemotherapy for testicular cancer - the cost of cure. Br. J. Cancer, GI: 479-84, 1990.
30. ZOROWKA, P. G.; SCHMITT, H. J. & GUTJAHR, P.-Evoked otoacoustic emissions and pure tone threshold audiometry in patients receiving cisplatinum therapy. Int. J. Pediatr. Otorhinolaryngol., 25: 73-80, 1993.
* Master Degree in Human Communication Disorders - Speech and Hearing Pathology at Universidade Federal de Sao Paulo - Escola Paulista de Medicina.
** Joint Professor, Ph.D., Head of the Discipline of Hearing Disorders of the Department of Otorhinolaryngology and Human Communication Disorders at
Universidade Federal de São Paulo - Escola Paulista de Medicina.
*** Professor, Ph.D., Head of the Sector of Pediatric Oncology of the Department of Pediatrics at Universidade Federal de Sao Paulo - Escola Paulista de Medicina.
**** Joint Professor (Retired) of the Department of Preventive Medicine at Universidade Federal de Sao Paulo - Escola Paulista de Medicina.
***** Joint Professor, Ph.D., Department of 0torhinolaryngology and Human Communication Disorders at Universidade Federal de Sao Paulo -Escola Paulista de Medicina.
Affiliation: Discipline of Hearing Disorders, Universidade Federal de Sao Paulo - Escola Paulista de Medicina.
Study presented at XI Congress of the American Academy of Audiology, on April 30, 1999, in Miami - USA.
Address for correspondence: Andrea Dishtehekenian - Rua do Acre, 729-03181-1000 Sao Paulo/ SP - Tel: (55 11) 6604-2165 - E-mail: andreadi@terra.crom.br
Article submitted on October 16, 1999. Article accepted on July 13. 2000.


